Low-dose bafilomycin attenuates neuronal cell death associated with autophagy-lysosome pathway dysfunction
- PMID: 20534000
- PMCID: PMC2910188
- DOI: 10.1111/j.1471-4159.2010.06838.x
Low-dose bafilomycin attenuates neuronal cell death associated with autophagy-lysosome pathway dysfunction
Abstract
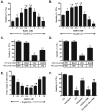
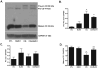
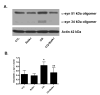
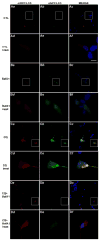
We have shown previously that the plecomacrolide antibiotics bafilomycin A1 and B1 significantly attenuate cerebellar granule neuron death resulting from agents that disrupt lysosome function. To further characterize bafilomycin-mediated cytoprotection, we examined its ability to attenuate the death of naïve and differentiated neuronal SH-SY5Y human neuroblastoma cells from agents that induce lysosome dysfunction in vitro, and from in vivo dopaminergic neuron death in C. elegans. Low-dose bafilomycin significantly attenuated SH-SY5Y cell death resulting from treatment with chloroquine, hydroxychloroquine amodiaquine and staurosporine. Bafilomycin also attenuated the chloroquine-induced reduction in processing of cathepsin D, the principal lysosomal aspartic acid protease, to its mature 'active' form. Chloroquine induced autophagic vacuole accumulation and inhibited autophagic flux, effects that were attenuated upon treatment with bafilomycin and were associated with a significant decrease in chloroquine-induced accumulation of detergent-insoluble alpha-synuclein oligomers. In addition, bafilomycin significantly and dose-dependently attenuated dopaminergic neuron death in C. elegans resulting from in vivo over-expression of human wild-type alpha-synuclein. Together, our findings suggest that low-dose bafilomycin is cytoprotective in part through its maintenance of the autophagy-lysosome pathway, and underscores its therapeutic potential for treating Parkinson's disease and other neurodegenerative diseases that exhibit disruption of protein degradation pathways and accumulation of toxic protein species.
Figures



References
-
- Anglade P, Vyas S, Javoy-Agid F, et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol. 1997;12(1):25–31. - PubMed
-
- Basque J, Martel M, Leduc R, Cantin AM. Lysosomotropic drugs inhibit maturation of transforming growth factor-beta. Can J Physiol Pharmacol. 2008;86(9):606–612. - PubMed
-
- Bidere N, Lorenzo HK, Carmona S, Laforge M, Harper F, Dumont C, Senik A. Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J Biol Chem. 2003 Aug 15;278(33):31401–31411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

