Energy conversion in natural and artificial photosynthesis
- PMID: 20534342
- PMCID: PMC2891097
- DOI: 10.1016/j.chembiol.2010.05.005
Energy conversion in natural and artificial photosynthesis
Abstract
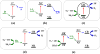
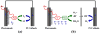
Modern civilization is dependent upon fossil fuels, a nonrenewable energy source originally provided by the storage of solar energy. Fossil-fuel dependence has severe consequences, including energy security issues and greenhouse gas emissions. The consequences of fossil-fuel dependence could be avoided by fuel-producing artificial systems that mimic natural photosynthesis, directly converting solar energy to fuel. This review describes the three key components of solar energy conversion in photosynthesis: light harvesting, charge separation, and catalysis. These processes are compared in natural and in artificial systems. Such a comparison can assist in understanding the general principles of photosynthesis and in developing working devices, including photoelectrochemical cells, for solar energy conversion.
2010 Elsevier Ltd. All rights reserved.
Figures

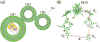

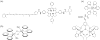
References
-
- Abrahamsson MLA, Baudin HB, Tran A, Philouze C, Berg KE, Raymond-Johansson MK, Sun L, Aakermark B, Styring S, Hammarström L. Ruthenium-manganese complexes for artificial photosynthesis: Factors controlling intramolecular electron transfer and excited-state quenching reactions. Inorg Chem. 2002;41:1534–1544. - PubMed
-
- Adir N. Elucidation of the molecular structures of components of the phycobilisome: reconstructing a giant. Photosynth Res. 2005;85:15–32. - PubMed
-
- Ahrens MJ, Sinks LE, Rybtchinski B, Liu W, Jones BA, Giaimo JM, Gusev AV, Goshe AJ, Tiede DM, Wasielewski MR. Self-assembly of supramolecular light-harvesting arrays from covalent multi-chromophore perylene-3,4:9,10-bis(dicarboximide) building blocks. J Am Chem Soc. 2004;126:8284–8294. - PubMed
-
- Alstrum-Acevedo JH, Brennaman MK, Meyer TJ. Chemical Approaches to Artificial Photosynthesis.2. Inorg Chem. 2005;44:6802–6827. - PubMed
-
- Ardo S, Meyer GJ. Photodriven heterogeneous charge transfer with transition-metal compounds anchored to TiO2 semiconductor surfaces. Chem Soc Rev. 2009;38:115–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

