Virtual ligand screening of the p300/CBP histone acetyltransferase: identification of a selective small molecule inhibitor
- PMID: 20534345
- PMCID: PMC2884008
- DOI: 10.1016/j.chembiol.2010.03.006
Virtual ligand screening of the p300/CBP histone acetyltransferase: identification of a selective small molecule inhibitor
Abstract
The histone acetyltransferase (HAT) p300/CBP is a transcriptional coactivator implicated in many gene regulatory pathways and protein acetylation events. Although p300 inhibitors have been reported, a potent, selective, and readily available active-site-directed small molecule inhibitor is not yet known. Here we use a structure-based, in silico screening approach to identify a commercially available pyrazolone-containing small molecule p300 HAT inhibitor, C646. C646 is a competitive p300 inhibitor with a K(i) of 400 nM and is selective versus other acetyltransferases. Studies on site-directed p300 HAT mutants and synthetic modifications of C646 confirm the importance of predicted interactions in conferring potency. Inhibition of histone acetylation and cell growth by C646 in cells validate its utility as a pharmacologic probe and suggest that p300/CBP HAT is a worthy anticancer target.
2010 Elsevier Ltd. All rights reserved.
Figures

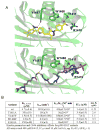
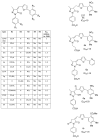


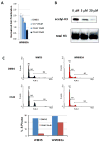

Comment in
-
HAT trick: p300, small molecule, inhibitor.Chem Biol. 2010 May 28;17(5):417-8. doi: 10.1016/j.chembiol.2010.05.002. Chem Biol. 2010. PMID: 20534339
References
-
- Abagyan R, Totrov M, Kuznetsov D. ICM-a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J Comput Chem. 1994;15:488–506.
-
- Abagyan R, Totrov M, Kuznetsov D. ICM-a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J Comput Chem. 1994;15:488–506.
-
- Arif M, Pradhan SK, Thanuja GR, Vedamurthy BM, Agrawal S, Dasgupta D, Kundu TK. Mechanism of p300 specific histone acetyltransferase inhibition by small molecules. J Med Chem. 2009;52:267–277. - PubMed
-
- Balasubramanyam K, Swaminathan V, Ranganathan A, Kundu TK. Small molecule modulators of histone acetyltransferase p300. J Biol Chem. 2003;278:19134–19140. - PubMed
-
- Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, Kundu TK. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004;279:51163–51171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

