Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling
- PMID: 20534436
- PMCID: PMC2895055
- DOI: 10.1073/pnas.1000751107
Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling
Abstract
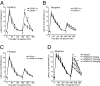
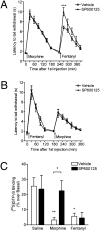
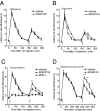
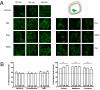
Ligand-directed signaling has been suggested as a basis for the differences in responses evoked by otherwise receptor-selective agonists. The underlying mechanisms are not understood, yet clearer definition of this concept may be helpful in the development of novel, pathway-selective therapeutic agents. We previously showed that kappa-opioid receptor activation of JNK by one class of ligand, but not another, caused persistent receptor inactivation. In the current study, we found that the mu-opioid receptor (MOR) could be similarly inactivated by a specific ligand class including the prototypical opioid, morphine. Acute analgesic tolerance to morphine and related opioids (morphine-6-glucuronide and buprenorphine) was blocked by JNK inhibition, but not by G protein receptor kinase 3 knockout. In contrast, a second class of mu-opioids including fentanyl, methadone, and oxycodone produced acute analgesic tolerance that was blocked by G protein receptor kinase 3 knockout, but not by JNK inhibition. Acute MOR desensitization, demonstrated by reduced D-Ala(2)-Met(5)-Glyol-enkephalin-stimulated [(35)S]GTPgammaS binding to spinal cord membranes from morphine-pretreated mice, was also blocked by JNK inhibition; however, desensitization of D-Ala(2)-Met(5)-Glyol-enkephalin-stimulated [(35)S]GTPgammaS binding following fentanyl pretreatment was not blocked by JNK inhibition. JNK-mediated receptor inactivation of the kappa-opioid receptor was evident in both agonist-stimulated [(35)S]GTPgammaS binding and opioid analgesic assays; however, gene knockout of JNK 1 selectively blocked kappa-receptor inactivation, whereas deletion of JNK 2 selectively blocked MOR inactivation. These findings suggest that ligand-directed activation of JNK kinases may generally provides an alternate mode of G protein-coupled receptor regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Berg KA, et al. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: Evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. - PubMed
-
- Drake MT, et al. beta-Arrestin-biased agonism at the beta2-adrenergic receptor. J Biol Chem. 2008;283:5669–5676. - PubMed
-
- Galandrin S, et al. Conformational rearrangements and signaling cascades involved in ligand-biased mitogen-activated protein kinase signaling through the beta1-adrenergic receptor. Mol Pharmacol. 2008;74:162–172. - PubMed
-
- Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. - PubMed
-
- Kenakin T. Functional selectivity through protean and biased agonism: Who steers the ship? Mol Pharmacol. 2007;72:1393–1401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

