Interactions between DSIF (DRB sensitivity inducing factor), NELF (negative elongation factor), and the Drosophila RNA polymerase II transcription elongation complex
- PMID: 20534440
- PMCID: PMC2895096
- DOI: 10.1073/pnas.1000681107
Interactions between DSIF (DRB sensitivity inducing factor), NELF (negative elongation factor), and the Drosophila RNA polymerase II transcription elongation complex
Abstract
Negative elongation factor (NELF) and 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole sensitivity-inducing factor (DSIF) are involved in pausing RNA Polymerase II (Pol II) in the promoter-proximal region of the hsp70 gene in Drosophila, before heat shock induction. Such blocks in elongation are widespread in the Drosophila genome. However, the mechanism by which DSIF and NELF participate in setting up the paused Pol II remains unclear. We analyzed the interactions among DSIF, NELF, and a reconstituted Drosophila Pol II elongation complex to gain insight into the mechanism of pausing. Our results show that DSIF and NELF require a nascent transcript longer than 18 nt to stably associate with the Pol II elongation complex. Protein-RNA cross-linking reveals that Spt5, the largest subunit of DSIF, contacts the nascent RNA as the RNA emerges from the elongation complex. Taken together, these results provide a possible model by which DSIF binds the elongation complex via association with the nascent transcript and subsequently recruits NELF. Although DSIF and NELF were both required for inhibition of transcription, we did not detect a NELF-RNA contact when the nascent transcript was between 22 and 31 nt long, which encompasses the region where promoter-proximal pausing occurs on many genes in Drosophila. This raises the possibility that RNA binding by NELF is not necessary in promoter-proximal pausing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
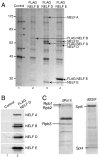
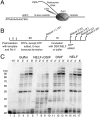
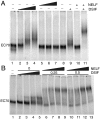
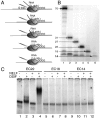

References
-
- Gilmour DS. Promoter proximal pausing on genes in metazoans. Chromosoma. 2009;118:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

