Mixed T cell receptor dimers harbor potentially harmful neoreactivity
- PMID: 20534461
- PMCID: PMC2890759
- DOI: 10.1073/pnas.1005802107
Mixed T cell receptor dimers harbor potentially harmful neoreactivity
Abstract
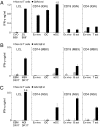
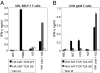
Adoptive transfer of T cell receptor (TCR)-transduced T cells may be an attractive strategy to target both hematological malignancies and solid tumors. By introducing a TCR, large numbers of T cells with defined antigen (Ag) specificity can be obtained. However, by introduction of a TCR, mixed TCR dimers can be formed. Besides the decrease in TCR expression of the introduced and endogenous TCR, these mixed TCR dimers could harbor potentially harmful specificities. In this study, we demonstrate that introduction of TCRs resulted in formation of neoreactive mixed TCR dimers, composed of the introduced TCR chains pairing with either the endogenous TCR alpha or beta chain. Neoreactivities observed were HLA class I or class II restricted. Most neoreactive mixed TCR dimers were allo-HLA reactive; however, neoreactive mixed TCR dimers with autoreactive activity were also observed. We demonstrate that inclusion of an extra disulfide bond between the constant domains of the introduced TCR markedly reduced neoreactivity, whereas enhanced effectiveness of the introduced TCR was observed. In conclusion, TCR transfer results in the formation of neoreactive mixed TCR dimers with the potential to generate off-target effects, underlining the importance of searching for techniques to facilitate preferential pairing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Collins RH, Jr, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–444. - PubMed
-
- Kolb HJ, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. - PubMed
-
- Clay TM, et al. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

