A mouse model of chondrocyte-specific somatic mutation reveals a role for Ext1 loss of heterozygosity in multiple hereditary exostoses
- PMID: 20534475
- PMCID: PMC2890731
- DOI: 10.1073/pnas.0914642107
A mouse model of chondrocyte-specific somatic mutation reveals a role for Ext1 loss of heterozygosity in multiple hereditary exostoses
Abstract
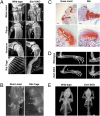
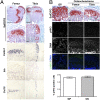
Multiple hereditary exostoses (MHE) is one of the most common skeletal dysplasias, exhibiting the formation of multiple cartilage-capped bony protrusions (osteochondroma) and characteristic bone deformities. Individuals with MHE carry heterozygous loss-of-function mutations in Ext1 or Ext2, genes which together encode an enzyme essential for heparan sulfate synthesis. Despite the identification of causative genes, the pathogenesis of MHE remains unclear, especially with regard to whether osteochondroma results from loss of heterozygosity of the Ext genes. Hampering elucidation of the pathogenic mechanism of MHE, both Ext1(+/-) and Ext2(+/-) heterozygous mutant mice, which mimic the genetic status of human MHE, are highly resistant to osteochondroma formation, especially in long bones. To address these issues, we created a mouse model in which Ext1 is stochastically inactivated in a chondrocyte-specific manner. We show that these mice develop multiple osteochondromas and characteristic bone deformities in a pattern and a frequency that are almost identical to those of human MHE, suggesting a role for Ext1 LOH in MHE. Surprisingly, however, genotyping and fate mapping analyses reveal that chondrocytes constituting osteochondromas are mixtures of mutant and wild-type cells. Moreover, osteochondromas do not possess many typical neoplastic properties. Together, our results suggest that inactivation of Ext1 in a small fraction of chondrocytes is sufficient for the development of osteochondromas and other skeletal defects associated with MHE. Because the observed osteochondromas in our mouse model do not arise from clonal growth of chondrocytes, they cannot be considered true neoplasms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
A mouse model of osteochondromagenesis from clonal inactivation of Ext1 in chondrocytes.Proc Natl Acad Sci U S A. 2010 Feb 2;107(5):2054-9. doi: 10.1073/pnas.0910875107. Epub 2009 Dec 22. Proc Natl Acad Sci U S A. 2010. PMID: 20080592 Free PMC article.
-
Palovarotene Inhibits Osteochondroma Formation in a Mouse Model of Multiple Hereditary Exostoses.J Bone Miner Res. 2018 Apr;33(4):658-666. doi: 10.1002/jbmr.3341. Epub 2017 Nov 30. J Bone Miner Res. 2018. PMID: 29120519 Free PMC article.
-
Signaling systems affecting the severity of multiple osteochondromas.Bone. 2018 Jun;111:71-81. doi: 10.1016/j.bone.2018.03.010. Epub 2018 Mar 13. Bone. 2018. PMID: 29545125
-
The pathogenic roles of heparan sulfate deficiency in hereditary multiple exostoses.Matrix Biol. 2018 Oct;71-72:28-39. doi: 10.1016/j.matbio.2017.12.011. Epub 2017 Dec 24. Matrix Biol. 2018. PMID: 29277722 Free PMC article. Review.
-
Multiple osteochondromas: mutation update and description of the multiple osteochondromas mutation database (MOdb).Hum Mutat. 2009 Dec;30(12):1620-7. doi: 10.1002/humu.21123. Hum Mutat. 2009. PMID: 19810120 Review.
Cited by
-
Genetic alterations in chondrosarcomas - keys to targeted therapies?Cell Oncol (Dordr). 2014 Apr;37(2):95-105. doi: 10.1007/s13402-014-0166-8. Epub 2014 Jan 24. Cell Oncol (Dordr). 2014. PMID: 24458248 Review.
-
Fibronectin and stem cell differentiation - lessons from chondrogenesis.J Cell Sci. 2012 Aug 15;125(Pt 16):3703-12. doi: 10.1242/jcs.095786. Epub 2012 Sep 12. J Cell Sci. 2012. PMID: 22976308 Free PMC article. Review.
-
Hereditary Multiple Exostoses: New Insights into Pathogenesis, Clinical Complications, and Potential Treatments.Curr Osteoporos Rep. 2017 Jun;15(3):142-152. doi: 10.1007/s11914-017-0355-2. Curr Osteoporos Rep. 2017. PMID: 28466453 Free PMC article. Review.
-
Osteoblastic heparan sulfate regulates osteoprotegerin function and bone mass.JCI Insight. 2018 Feb 8;3(3):e89624. doi: 10.1172/jci.insight.89624. eCollection 2018 Feb 8. JCI Insight. 2018. PMID: 29415886 Free PMC article.
-
Glycobiology and the growth plate: current concepts in multiple hereditary exostoses.J Pediatr Orthop. 2011 Jul-Aug;31(5):577-86. doi: 10.1097/BPO.0b013e31821c7738. J Pediatr Orthop. 2011. PMID: 21654469 Free PMC article. Review.
References
-
- Erol B, Dormans JP, States L, Kaplan FS. Skeletal dysplasias and metabolic disorders of bone. In: Dormans JP, editor. Pediatric Orthopaedics and Sports Medicine: The Requisites in Pediatrics. St. Louis: Mosby; 2004.
-
- Stieber JR, Dormans JP. Manifestations of hereditary multiple exostoses. J Am Acad Orthop Surg. 2005;13:110–120. - PubMed
-
- Fogel GR, McElfresh EC, Peterson HA, Wicklund PT. Management of deformities of the forearm in multiple hereditary osteochondromas. J Bone Joint Surg Am. 1984;66:670–680. - PubMed
-
- Oestreich AT, Huslig EL. Hereditary multiple exostosis: another etiology of short leg and scoliosis. J Manipulative Physiol Ther. 1985;8:267–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

