Imaging of enzyme replacement therapy using PET
- PMID: 20534487
- PMCID: PMC2890769
- DOI: 10.1073/pnas.1003247107
Imaging of enzyme replacement therapy using PET
Abstract
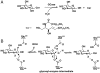
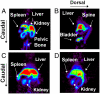
Direct enzyme replacement therapy (ERT) has been introduced as a means to treat a number of rare, complex genetic conditions associated with lysosomal dysfunction. Gaucher disease was the first for which this therapy was applied and remains the prototypical example. Although ERT using recombinant lysosomal enzymes has been shown to be effective in altering the clinical course of Gaucher disease, Fabry disease, Hurler syndrome, Hunter syndrome, Maroteaux-Lamy syndrome, and Pompe disease, the recalcitrance of certain disease manifestations underscores important unanswered questions related to dosing regimes, tissue half-life of the recombinant enzyme and the ability of intravenously administered enzyme to reach critical sites of known disease pathology. We have developed an innovative method for tagging acid beta-glucocerebrosidase (GCase), the recombinant enzyme formulated in Cerezyme(R) used to treat Gaucher disease, using an (18)F-labeled substrate analogue that becomes trapped within the active site of the enzyme. Using micro-PET we show that the tissue distribution of injected enzyme can be imaged in a murine model and that the PET data correlate with tissue (18)F counts. Further we show that PET imaging readily monitors pharmacokinetic changes effected by receptor blocking. The ability to (18)F-label GCase to monitor the enzyme distribution and tissue half-life in vivo by PET provides a powerful research tool with an immediate clinical application to Gaucher disease and a clear path for application to other ERTs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rohrbach M, Clarke JTR. Treatment of lysosomal storage disorders—progress with enzyme replacement therapy. Drugs. 2007;67:2697–2716. - PubMed
-
- Liou B, et al. Analyses of variant acid beta-glucosidases—effects of Gaucher Disease mutations. J Biol Chem. 2006;281:4242–4253. - PubMed
-
- Fuller M, et al. Glucosylceramide accumulation is not confined to the lysosome in fibroblasts from patients with Gaucher Disease. Mol Genet Metab. 2008;93:437–443. - PubMed
-
- Kempton JB, Withers SG. Mechanism of Agrobacterium beta-glucosidase—kinetic studies. Biochemistry. 1992;31:9961–9969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

