Central role of the RNA polymerase trigger loop in intrinsic RNA hydrolysis
- PMID: 20534498
- PMCID: PMC2890756
- DOI: 10.1073/pnas.0914424107
Central role of the RNA polymerase trigger loop in intrinsic RNA hydrolysis
Abstract
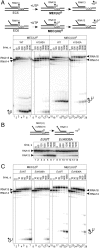
The active center of RNA polymerase can hydrolyze phosphodiester bonds in nascent RNA, a reaction thought to be important for proofreading of transcription. The reaction proceeds via a general two Mg(2+) mechanism and is assisted by the 3' end nucleotide of the transcript. Here, by using Thermus aquaticus RNA polymerase, we show that the reaction also requires the flexible domain of the active center, the trigger loop (TL). We show that the invariant histidine (beta' His1242) of the TL is essential for hydrolysis/proofreading and participates in the reaction in two distinct ways: by positioning the 3' end nucleotide of the transcript that assists catalysis and/or by directly participating in the reaction as a general base. We also show that participation of the beta' His1242 of the TL in phosphodiester bond hydrolysis does not depend on the extent of elongation complex backtracking. We obtained similar results with Escherichia coli RNA polymerase, indicating that the function of the TL in phosphodiester bond hydrolysis is conserved among bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Steitz TA. A mechanism for all polymerases. Nature. 1998;391(6664):231–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

