Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells
- PMID: 20534522
- PMCID: PMC2895108
- DOI: 10.1073/pnas.1001261107
Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells
Abstract
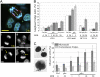
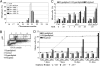
Escherichia coli is a normal inhabitant of the human gut. However, E. coli strains of phylogenetic group B2 harbor a genomic island called "pks" that codes for the production of a polyketide-peptide genotoxin, Colibactin. Here we report that in vivo infection with E. coli harboring the pks island, but not with a pks isogenic mutant, induced the formation of phosphorylated H2AX foci in mouse enterocytes. We show that a single, short exposure of cultured mammalian epithelial cells to live pks(+) E. coli at low infectious doses induced a transient DNA damage response followed by cell division with signs of incomplete DNA repair, leading to anaphase bridges and chromosome aberrations. Micronuclei, aneuploidy, ring chromosomes, and anaphase bridges persisted in dividing cells up to 21 d after infection, indicating occurrence of breakage-fusion-bridge cycles and chromosomal instability. Exposed cells exhibited a significant increase in gene mutation frequency and anchorage-independent colony formation, demonstrating the infection mutagenic and transforming potential. Therefore, colon colonization with these E. coli strains harboring the pks island could contribute to the development of sporadic colorectal cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Bacterial toxins: Escherichia coli damages host DNA.Nat Rev Microbiol. 2010 Aug;8(8):534. doi: 10.1038/nrmicro2414. Nat Rev Microbiol. 2010. PMID: 20665959 No abstract available.
References
-
- Nowrouzian FL, Wold AE, Adlerberth I. Escherichia coli strains belonging to phylogenetic group B2 have superior capacity to persist in the intestinal microflora of infants. J Infect Dis. 2005;191:1078–1083. - PubMed
-
- Nougayrède JP, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848–851. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

