Alterations of cortical pyramidal neurons in mice lacking high-affinity nicotinic receptors
- PMID: 20534523
- PMCID: PMC2895077
- DOI: 10.1073/pnas.1006269107
Alterations of cortical pyramidal neurons in mice lacking high-affinity nicotinic receptors
Abstract
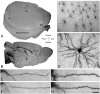
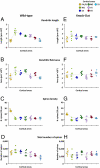
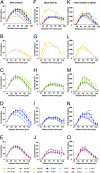
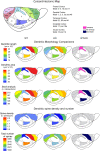
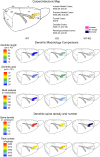
The neuronal nicotinic acetylcholine receptors (nAChRs) are allosteric membrane proteins involved in multiple cognitive processes, including attention, learning, and memory. The most abundant form of heterooligomeric nAChRs in the brain contains the beta2- and alpha4- subunits and binds nicotinic agonists with high affinity. In the present study, we investigated in the mouse the consequences of the deletion of one of the nAChR components: the beta2-subunit (beta2(-/-)) on the microanatomy of cortical pyramidal cells. Using an intracellular injection method, complete basal dendritic arbors of 650 layer III pyramidal neurons were sampled from seven cortical fields, including primary sensory, motor, and associational areas, in both beta2(-/-) and WT animals. We observed that the pyramidal cell phenotype shows significant quantitative differences among different cortical areas in mutant and WT mice. In WT mice, the density of dendritic spines was rather similar in all cortical fields, except in the prelimbic/infralimbic cortex, where it was significantly higher. In the absence of the beta2-subunit, the most significant reduction in the density of spines took place in this high-order associational field. Our data suggest that the beta2-subunit is involved in the dendritic morphogenesis of pyramidal neurons and, in particular, in the circuits that contribute to the high-order functional connectivity of the cerebral cortex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
High-Affinity Nicotinic Receptors Modulate Spontaneous Cortical Up States In Vitro.J Neurosci. 2015 Aug 12;35(32):11196-208. doi: 10.1523/JNEUROSCI.5222-14.2015. J Neurosci. 2015. PMID: 26269630 Free PMC article.
-
Premature Aging Phenotype in Mice Lacking High-Affinity Nicotinic Receptors: Region-Specific Changes in Layer V Pyramidal Cell Morphology.Cereb Cortex. 2015 Aug;25(8):2138-48. doi: 10.1093/cercor/bhu019. Epub 2014 Feb 18. Cereb Cortex. 2015. PMID: 24554727
-
Sez-6 proteins affect dendritic arborization patterns and excitability of cortical pyramidal neurons.Neuron. 2007 Nov 21;56(4):621-39. doi: 10.1016/j.neuron.2007.09.018. Neuron. 2007. PMID: 18031681
-
Identification of four classes of brain nicotinic receptors using beta2 mutant mice.J Neurosci. 1998 Jun 15;18(12):4461-72. doi: 10.1523/JNEUROSCI.18-12-04461.1998. J Neurosci. 1998. PMID: 9614223 Free PMC article.
-
Long distance projections of cortical pyramidal neurons.J Neurosci Res. 2018 Sep;96(9):1467-1475. doi: 10.1002/jnr.23978. Epub 2016 Nov 12. J Neurosci Res. 2018. PMID: 27862192 Free PMC article. Review.
Cited by
-
Branching angles of pyramidal cell dendrites follow common geometrical design principles in different cortical areas.Sci Rep. 2014 Aug 1;4:5909. doi: 10.1038/srep05909. Sci Rep. 2014. PMID: 25081193 Free PMC article.
-
Segmentation of Neurons from Fluorescence Calcium Recordings Beyond Real-time.Nat Mach Intell. 2021 Jul;3(7):590-600. doi: 10.1038/s42256-021-00342-x. Epub 2021 May 20. Nat Mach Intell. 2021. PMID: 34485824 Free PMC article.
-
Regulation of the actin cytoskeleton in dendritic spines.Adv Exp Med Biol. 2012;970:81-95. doi: 10.1007/978-3-7091-0932-8_4. Adv Exp Med Biol. 2012. PMID: 22351052 Free PMC article. Review.
-
Variation in Pyramidal Cell Morphology Across the Human Anterior Temporal Lobe.Cereb Cortex. 2021 Jul 5;31(8):3592-3609. doi: 10.1093/cercor/bhab034. Cereb Cortex. 2021. PMID: 33723567 Free PMC article.
-
Early Signs of Pathological Cognitive Aging in Mice Lacking High-Affinity Nicotinic Receptors.Front Aging Neurosci. 2016 Apr 27;8:91. doi: 10.3389/fnagi.2016.00091. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27199738 Free PMC article.
References
-
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. - PubMed
-
- Changeux JP, Edelstein SJ. Nicotinic Acetylcholine Receptors: From Molecular Biology to Cognition. Paris: Odile Jacob; 2005.
-
- Changeux JP, et al. Brain nicotinic receptors: Structure and regulation, role in learning and reinforcement. Brain Res Brain Res Rev. 1998;26:198–216. - PubMed
-
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berl) 1998;138:217–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

