Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation
- PMID: 20534530
- PMCID: PMC2895126
- DOI: 10.1073/pnas.1002099107
Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation
Abstract
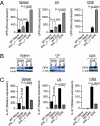
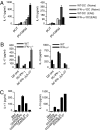
Dendritic cells (DCs) play a central role in determining the induction of T cell responses. IL-27 production by DCs favors induction of IL-10-producing regulatory T cells, whereas osteopontin (OPN) promotes pathogenic IL-17 T cell responses. The regulatory mechanisms in DCs that control these two cells types are not understood well. Here, we show that IFN-gamma induces IL-27 while inhibiting OPN expression in DCs both in vitro and in vivo and that engagement of IFN-gammaR expressed by DCs leads to suppression of IL-17 production while inducing IL-10 from T cells. DCs modified by IFN-gamma acquire IL-27-dependent regulatory function, promote IL-10-mediated T cell tolerance, and suppress autoimmune inflammation. Thus, our results identify a previously unknown pathway by which IFN-gamma limits IL-17-mediated autoimmune inflammation through differential regulation of OPN and IL-27 expression in DCs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: Related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. - PubMed
-
- Pflanz S, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. - PubMed
-
- Takeda A, et al. Cutting edge: Role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. - PubMed
-
- Yoshimoto T, Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: A novel therapeutic way for Th2–mediated allergic inflammation. J Immunol. 2007;179:4415–4423. - PubMed
-
- Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J Immunol. 2007;179:6421–6428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

