Identification of a protein required for recovery of full antenna capacity in OCP-related photoprotective mechanism in cyanobacteria
- PMID: 20534537
- PMCID: PMC2895087
- DOI: 10.1073/pnas.1002912107
Identification of a protein required for recovery of full antenna capacity in OCP-related photoprotective mechanism in cyanobacteria
Abstract
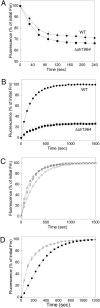
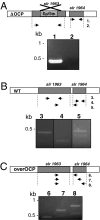
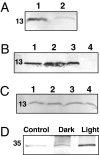
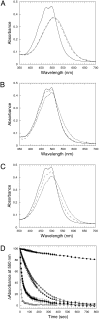
High light can be lethal for photosynthetic organisms. Similar to plants, most cyanobacteria protect themselves from high irradiance by increasing thermal dissipation of excess absorbed energy. The photoactive soluble orange carotenoid protein (OCP) is essential for the triggering of this photoprotective mechanism. Light induces structural changes in the carotenoid and the protein, leading to the formation of a red active form. Through targeted gene interruption we have now identified a protein that mediates the recovery of the full antenna capacity when irradiance decreases. In Synechocystis PCC 6803, this protein, which we called the fluorescence recovery protein (FRP), is encoded by the slr1964 gene. Homologues of this gene are present in all of the OCP-containing strains. The FRP is a 14-kDa protein, strongly attached to the membrane, which interacts with the active red form of the OCP. In vitro this interaction greatly accelerates the conversion of the red OCP form to the orange form. We propose that in vivo, FRP plays a key role in removing the red OCP from the phycobilisome and in the conversion of the free red OCP to the orange inactive form. The discovery of FRP and its characterization are essential elements in the understanding of the OCP-related photoprotective mechanism in cyanobacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. - PubMed
-
- Niyogi KK. Photoprotection revisited: Genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–359. - PubMed
-
- Pascal AA, et al. Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature. 2005;436:134–137. - PubMed
-
- Ruban AV, et al. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature. 2007;450:575–578. - PubMed
-
- Adir N. Elucidation of the molecular structures of components of the phycobilisome: Reconstructing a giant. Photosynth Res. 2005;85:15–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

