SH2 domain containing leukocyte phosphoprotein of 76-kDa (SLP-76) feedback regulation of ZAP-70 microclustering
- PMID: 20534575
- PMCID: PMC2890474
- DOI: 10.1073/pnas.0909112107
SH2 domain containing leukocyte phosphoprotein of 76-kDa (SLP-76) feedback regulation of ZAP-70 microclustering
Abstract
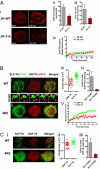
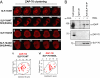
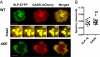
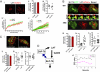
T cell receptor (TCR) signaling involves CD4/CD8-p56lck recruitment of ZAP-70 to the TCR receptor, ZAP-70 phosphorylation of LAT that is followed by LAT recruitment of the GADS-SLP-76 complex. Back regulation of ZAP-70 by SLP-76 has not been documented. In this paper, we show that anti-CD3 induced ZAP-70 cluster formation is significantly reduced in the absence of SLP-76 (i.e., J14 cells) and in the presence of a mutant of SLP-76 (4KE) in Jurkat and primary T cells. Both the number of cells with clusters and the number of clusters per cell were reduced. This effect was not mediated by SLP-76 SH2 domain binding to ZAP-70 because SLP-76 failed to precipitate ZAP-70 and an inactivating SH2 domain mutation (i.e., R448L) on SLP-76 4KE did not reverse the inhibition of ZAP-70 clustering. Mutation of R448 on WT SLP-76 still supported ZAP-70 clustering. Intriguingly, by contrast, LAT clustering occurred normally in the absence of SLP-76, or the presence of 4KE SLP-76 indicating that this transmembrane adaptor can operate independently of ZAP-70-GADS-SLP-76. Our findings reconfigure the TCR signaling pathway by showing SLP-76 back-regulation of ZAP-70, an event that could ensure that signaling components are in balance for optimal T cell activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Samelson LE. Signal transduction mediated by the T cell antigen receptor: The role of adapter proteins. Annu Rev Immunol. 2002;20:371–394. - PubMed
-
- Rudd CE. Adaptors and molecular scaffolds in immune cell signaling. Cell. 1999;96:5–8. - PubMed
-
- Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat Immunol. 2003;4:110–116. - PubMed
-
- Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: The ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. - PubMed
-
- Berry DM, Nash P, Liu SK, Pawson T, McGlade CJ. A high-affinity Arg-X-X-Lys SH3 binding motif confers specificity for the interaction between Gads and SLP-76 in T cell signaling. Curr Biol. 2002;12:1336–1341. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

