Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex
- PMID: 20534583
- PMCID: PMC2919089
- DOI: 10.1074/jbc.M110.145953
Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex
Abstract
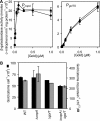
Salmonella enterica sv. typhimurium (S. enterica sv. Typhimurium) has two metal-transporting P(1)-type ATPases whose actions largely overlap with respect to growth in elevated copper. Mutants lacking both ATPases over-accumulate copper relative to wild-type or either single mutant. Such duplication of ATPases is unusual in bacterial copper tolerance. Both ATPases are under the control of MerR family metal-responsive transcriptional activators. Analyses of periplasmic copper complexes identified copper-CueP as one of the predominant metal pools. Expression of cueP was recently shown to be controlled by the same metal-responsive activator as one of the P(1)-type ATPase genes (copA), and copper-CueP is a further atypical feature of copper homeostasis in S. enterica sv. Typhimurium. Elevated copper is detected by a reporter construct driven by the promoter of copA in wild-type S. enterica sv. Typhimurium during infection of macrophages. Double mutants missing both ATPases also show reduced survival inside cultured macrophages. It is hypothesized that elevated copper within macrophages may have selected for specialized copper-resistance systems in pathogenic microorganism such as S. enterica sv. Typhimurium.
Figures



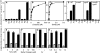

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

