Der p 5 crystal structure provides insight into the group 5 dust mite allergens
- PMID: 20534590
- PMCID: PMC2919102
- DOI: 10.1074/jbc.M110.128306
Der p 5 crystal structure provides insight into the group 5 dust mite allergens
Abstract
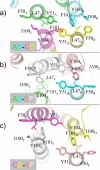
Group 5 allergens from house dust mites elicit strong IgE antibody binding in mite-allergic patients. The structure of Der p 5 was determined by x-ray crystallography to better understand the IgE epitopes, to investigate the biologic function in mites, and to compare with the conflicting published Blo t 5 structures, designated 2JMH and 2JRK in the Protein Data Bank. Der p 5 is a three-helical bundle similar to Blo t 5, but the interactions of the helices are more similar to 2JMH than 2JRK. The crystallographic asymmetric unit contains three dimers of Der p 5 that are not exactly alike. Solution scattering techniques were used to assess the multimeric state of Der p 5 in vitro and showed that the predominant state was monomeric, similar to Blo t 5, but larger multimeric species are also present. In the crystal, the formation of the Der p 5 dimer creates a large hydrophobic cavity of approximately 3000 A(3) that could be a ligand-binding site. Many allergens are known to bind hydrophobic ligands, which are thought to stimulate the innate immune system and have adjuvant-like effects on IgE-mediated inflammatory responses.
Figures





Similar articles
-
Lack of human IgE cross-reactivity between mite allergens Blo t 1 and Der p 1.Allergy. 2003 Sep;58(9):912-20. doi: 10.1034/j.1398-9995.2003.00215.x. Allergy. 2003. PMID: 12911421
-
Characterization of a hybrid protein designed with segments of allergens from Blomia tropicalis and Dermatophagoides pteronyssinus.Immunol Lett. 2018 Apr;196:103-112. doi: 10.1016/j.imlet.2018.01.012. Epub 2018 Feb 3. Immunol Lett. 2018. PMID: 29408409
-
NMR structure and IgE epitopes of Blo t 21, a major dust mite allergen from Blomia tropicalis.J Biol Chem. 2012 Oct 5;287(41):34776-85. doi: 10.1074/jbc.M112.348730. Epub 2012 Aug 10. J Biol Chem. 2012. PMID: 22887997 Free PMC article.
-
The structure of the dust mite allergen Der p 7 reveals similarities to innate immune proteins.J Allergy Clin Immunol. 2010 Apr;125(4):909-917.e4. doi: 10.1016/j.jaci.2009.12.016. Epub 2010 Mar 11. J Allergy Clin Immunol. 2010. PMID: 20226507 Free PMC article.
-
[Allergens from Dermatophagoides dust mites: origin, antigenic and structural characteristics, and therapeutic agents].Biokhimiia. 1995 Feb;60(2):218-37. Biokhimiia. 1995. PMID: 7718666 Review. Russian.
Cited by
-
Higher Wheal Sizes of Dermatophagoides farinae Sensitization Exhibit Worse Nasal Symptoms in Allergic Rhinitis Patients.Front Med (Lausanne). 2022 Feb 28;9:843432. doi: 10.3389/fmed.2022.843432. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35295602 Free PMC article.
-
Der f 21, a novel allergen from dermatophagoides farina.Am J Transl Res. 2016 Jan 15;8(1):49-59. eCollection 2016. Am J Transl Res. 2016. PMID: 27069539 Free PMC article.
-
Dermatophagoides pteronyssinus proteins and their role in the diagnostics and management of house dust mite allergy: exploring allergenic components.Postepy Dermatol Alergol. 2024 Aug;41(4):339-349. doi: 10.5114/ada.2024.142390. Epub 2024 Aug 21. Postepy Dermatol Alergol. 2024. PMID: 39290903 Free PMC article. Review.
-
Serological, genomic and structural analyses of the major mite allergen Der p 23.Clin Exp Allergy. 2016 Feb;46(2):365-76. doi: 10.1111/cea.12680. Clin Exp Allergy. 2016. PMID: 26602749 Free PMC article.
-
100 Years later: Celebrating the contributions of x-ray crystallography to allergy and clinical immunology.J Allergy Clin Immunol. 2015 Jul;136(1):29-37.e10. doi: 10.1016/j.jaci.2015.05.016. J Allergy Clin Immunol. 2015. PMID: 26145985 Free PMC article. Review.
References
-
- Sporik R., Chapman M. D., Platts-Mills T. A. (1992) Clin. Exp. Allergy 22, 897–906 - PubMed
-
- Chapman M. D., Pomés A., Breiteneder H., Ferreira F. (2007) J. Allergy Clin. Immunol. 119, 414–420 - PubMed
-
- Thomas W. R., Hales B. J. (2007) Immunol. Res. 37, 187–199 - PubMed
-
- Arruda L. K., Rizzo M. C., Chapman M. D., Fernandez-Caldas E., Baggio D., Platts-Mills T. A., Naspitz C. K. (1991) Clin. Exp. Allergy 21, 433–439 - PubMed
-
- Caraballo L., Puerta L., Fernández-Caldas E., Lockey R. F., Martínez B. (1998) J. Investig. Allergol. Clin. Immunol. 8, 281–284 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

