Myocardin-related transcription factors regulate the Cdk5/Pctaire1 kinase cascade to control neurite outgrowth, neuronal migration and brain development
- PMID: 20534669
- PMCID: PMC2889604
- DOI: 10.1242/dev.047605
Myocardin-related transcription factors regulate the Cdk5/Pctaire1 kinase cascade to control neurite outgrowth, neuronal migration and brain development
Abstract
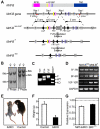
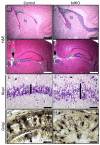
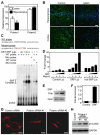
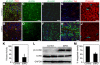
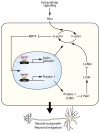
Numerous motile cell functions depend on signaling from the cytoskeleton to the nucleus. Myocardin-related transcription factors (MRTFs) translocate to the nucleus in response to actin polymerization and cooperate with serum response factor (Srf) to regulate the expression of genes encoding actin and other components of the cytoskeleton. Here, we show that MRTF-A (Mkl1) and MRTF-B (Mkl2) redundantly control neuronal migration and neurite outgrowth during mouse brain development. Conditional deletion of the genes encoding these Srf coactivators disrupts the formation of multiple brain structures, reflecting a failure in neuronal actin polymerization and cytoskeletal assembly. These abnormalities were accompanied by dysregulation of the actin-severing protein gelsolin and Pctaire1 (Cdk16) kinase, which cooperates with Cdk5 to initiate a kinase cascade that governs cytoskeletal rearrangements essential for neuron migration and neurite outgrowth. Thus, the MRTF/Srf partnership interlinks two key signaling pathways that control actin treadmilling and neuronal maturation, thereby fulfilling a regulatory loop that couples cytoskeletal dynamics to nuclear gene transcription during brain development.
Figures


Similar articles
-
Muscle-specific signaling mechanism that links actin dynamics to serum response factor.Mol Cell Biol. 2005 Apr;25(8):3173-81. doi: 10.1128/MCB.25.8.3173-3181.2005. Mol Cell Biol. 2005. PMID: 15798203 Free PMC article.
-
Identification of the intermediate filament protein synemin/SYNM as a target of myocardin family coactivators.Am J Physiol Cell Physiol. 2019 Dec 1;317(6):C1128-C1142. doi: 10.1152/ajpcell.00047.2019. Epub 2019 Aug 28. Am J Physiol Cell Physiol. 2019. PMID: 31461342
-
Redox modification of nuclear actin by MICAL-2 regulates SRF signaling.Cell. 2014 Jan 30;156(3):563-76. doi: 10.1016/j.cell.2013.12.035. Epub 2014 Jan 16. Cell. 2014. PMID: 24440334 Free PMC article.
-
Actin-mediated gene expression in neurons: the MRTF-SRF connection.Biol Chem. 2010 Jun;391(6):591-7. doi: 10.1515/BC.2010.061. Biol Chem. 2010. PMID: 20370316 Review.
-
Linking actin dynamics and gene transcription to drive cellular motile functions.Nat Rev Mol Cell Biol. 2010 May;11(5):353-65. doi: 10.1038/nrm2890. Nat Rev Mol Cell Biol. 2010. PMID: 20414257 Free PMC article. Review.
Cited by
-
MKL1 and MKL2 play redundant and crucial roles in megakaryocyte maturation and platelet formation.Blood. 2012 Sep 13;120(11):2317-29. doi: 10.1182/blood-2012-04-420828. Epub 2012 Jul 17. Blood. 2012. PMID: 22806889 Free PMC article.
-
Synaptic localisation of SRF coactivators, MKL1 and MKL2, and their role in dendritic spine morphology.Sci Rep. 2018 Jan 15;8(1):727. doi: 10.1038/s41598-017-18905-7. Sci Rep. 2018. PMID: 29335431 Free PMC article.
-
Mechanisms of specificity in neuronal activity-regulated gene transcription.Prog Neurobiol. 2011 Aug;94(3):259-95. doi: 10.1016/j.pneurobio.2011.05.003. Epub 2011 May 18. Prog Neurobiol. 2011. PMID: 21620929 Free PMC article. Review.
-
Common variants in the MKL1 gene confer risk of schizophrenia.Schizophr Bull. 2015 May;41(3):715-27. doi: 10.1093/schbul/sbu156. Epub 2014 Nov 7. Schizophr Bull. 2015. PMID: 25380769 Free PMC article.
-
MKL1 inhibits cell cycle progression through p21 in podocytes.BMC Mol Biol. 2015 Feb 12;16(1):1. doi: 10.1186/s12867-015-0029-5. BMC Mol Biol. 2015. PMID: 25888165 Free PMC article.
References
-
- Ahlemeyer B., Baumgart-Vogt E. (2005). Optimized protocols for the simultaneous preparation of primary neuronal cultures of the neocortex, hippocampus and cerebellum from individual newborn (P0.5) C57Bl/6J mice. J. Neurosci. Methods 149, 110-120 - PubMed
-
- Arber S., Barbayannis F. A., Hanser H., Schneider C., Stanyon C. A., Bernard O., Caroni P. (1998). Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393, 805-809 - PubMed
-
- Ayala R., Shu T., Tsai L. H. (2007). Trekking across the brain: the journey of neuronal migration. Cell 128, 29-43 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

