Cell type-specific thalamic innervation in a column of rat vibrissal cortex
- PMID: 20534783
- PMCID: PMC2936808
- DOI: 10.1093/cercor/bhq069
Cell type-specific thalamic innervation in a column of rat vibrissal cortex
Abstract
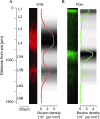
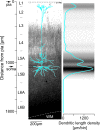
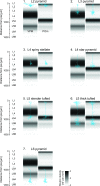
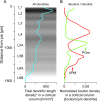
This is the concluding article in a series of 3 studies that investigate the anatomical determinants of thalamocortical (TC) input to excitatory neurons in a cortical column of rat primary somatosensory cortex (S1). We used viral synaptophysin-enhanced green fluorescent protein expression in thalamic neurons and reconstructions of biocytin-labeled cortical neurons in TC slices to quantify the number and distribution of boutons from the ventral posterior medial (VPM) and posteromedial (POm) nuclei potentially innervating dendritic arbors of excitatory neurons located in layers (L)2-6 of a cortical column in rat somatosensory cortex. We found that 1) all types of excitatory neurons potentially receive substantial TC input (90-580 boutons per neuron); 2) pyramidal neurons in L3-L6 receive dual TC input from both VPM and POm that is potentially of equal magnitude for thick-tufted L5 pyramidal neurons (ca. 300 boutons each from VPM and POm); 3) L3, L4, and L5 pyramidal neurons have multiple (2-4) subcellular TC innervation domains that match the dendritic compartments of pyramidal cells; and 4) a subtype of thick-tufted L5 pyramidal neurons has an additional VPM innervation domain in L4. The multiple subcellular TC innervation domains of L5 pyramidal neurons may partly explain their specific action potential patterns observed in vivo. We conclude that the substantial potential TC innervation of all excitatory neuron types in a cortical column constitutes an anatomical basis for the initial near-simultaneous representation of a sensory stimulus in different neuron types.
Figures




Comment in
-
Radial columns in cortical architecture: it is the composition that counts.Cereb Cortex. 2010 Oct;20(10):2261-4. doi: 10.1093/cercor/bhq127. Epub 2010 Jul 28. Cereb Cortex. 2010. PMID: 20667930 Free PMC article.
References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Ahissar E, Sosnik R, Haidarliu S. Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature. 2000;406:302–306. - PubMed
-
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992;68:1345–1358. - PubMed
-
- Bannister AP. Inter- and intra-laminar connections of pyramidal cells in the neocortex. Neurosci Res. 2005;53:95–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

