Number and laminar distribution of neurons in a thalamocortical projection column of rat vibrissal cortex
- PMID: 20534784
- PMCID: PMC2936806
- DOI: 10.1093/cercor/bhq067
Number and laminar distribution of neurons in a thalamocortical projection column of rat vibrissal cortex
Abstract
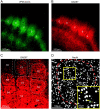
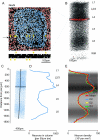
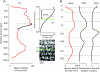
This is the second article in a series of three studies that investigate the anatomical determinants of thalamocortical (TC) input to excitatory neurons in a cortical column of rat primary somatosensory cortex (S1). Here, we report the number and distribution of NeuN-positive neurons within the C2, D2, and D3 TC projection columns in P27 rat somatosensory barrel cortex based on an exhaustive identification of 89,834 somata in a 1.15 mm(3) volume of cortex. A single column contained 19,109 ± 444 neurons (17,560 ± 399 when normalized to a standard-size projection column). Neuron density differences along the vertical column axis delineated "cytoarchitectonic" layers. The resulting neuron numbers per layer in the average column were 63 ± 10 (L1), 2039 ± 524 (L2), 3735 ± 905 (L3), 4447 ± 439 (L4), 1737 ± 251 (L5A), 2235 ± 99 (L5B), 3786 ± 168 (L6A), and 1066 ± 170 (L6B). These data were then used to derive the layer-specific action potential (AP) output of a projection column. The estimates confirmed previous reports suggesting that the ensembles of spiny L4 and thick-tufted pyramidal neurons emit the major fraction of APs of a column. The number of APs evoked in a column by a sensory stimulus (principal whisker deflection) was estimated as 4441 within 100 ms post-stimulus.
Figures
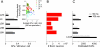
Comment in
-
Radial columns in cortical architecture: it is the composition that counts.Cereb Cortex. 2010 Oct;20(10):2261-4. doi: 10.1093/cercor/bhq127. Epub 2010 Jul 28. Cereb Cortex. 2010. PMID: 20667930 Free PMC article.
References
-
- Beaulieu C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res. 1993;609:284–292. - PubMed
-
- Beaulieu C, Kisvarday Z, Somogyi P, Cynader M, Cowey A. Quantitative distribution of GABA-immunopositive and -immunonegative neurons and synapses in the monkey striate cortex (area 17) Cereb Cortex. 1992;2:295–309. - PubMed
-
- Douglas RJ, Martin KA. Mapping the matrix: the ways of neocortex. Neuron. 2007;56:226–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

