Aspergillus RabB Rab5 integrates acquisition of degradative identity with the long distance movement of early endosomes
- PMID: 20534811
- PMCID: PMC2912360
- DOI: 10.1091/mbc.e10-02-0119
Aspergillus RabB Rab5 integrates acquisition of degradative identity with the long distance movement of early endosomes
Abstract
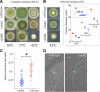
Aspergillus nidulans early endosomes display characteristic long-distance bidirectional motility. Simultaneous dual-channel acquisition showed that the two Rab5 paralogues RabB and RabA colocalize in these early endosomes and also in larger, immotile mature endosomes. However, RabB-GTP is the sole recruiter to endosomes of Vps34 PI3K (phosphatidylinositol-3-kinase) and the phosphatidylinositol-3-phosphate [PI(3)P] effector AnVps19 and rabB Delta, leading to thermosensitivity prevents multivesicular body sorting of endocytic cargo. Thus, RabB is the sole mediator of degradative endosomal identity. Importantly, rabB Delta, unlike rabA Delta, prevents early endosome movement. As affinity experiments and pulldowns showed that RabB-GTP recruits AnVps45, RabB coordinates PI(3)P-dependent endosome-to-vacuole traffic with incoming traffic from the Golgi and with long-distance endosomal motility. However, the finding that Anvps45 Delta, unlike rabB Delta, severely impairs growth indicates that AnVps45 plays RabB-independent functions. Affinity chromatography showed that the CORVET complex is a RabB and, to a lesser extent, a RabA effector, in agreement with GST pulldown assays of AnVps8. rabB Delta leads to smaller vacuoles, suggesting that it impairs homotypic vacuolar fusion, which would agree with the sequential maturation of endosomal CORVET into HOPS proposed for Saccharomyces cerevisiae. rabB Delta and rabA Delta mutations are synthetically lethal, demonstrating that Rab5-mediated establishment of endosomal identity is essential for A. nidulans.
Figures









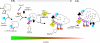
References
-
- Abenza J. F., Pantazopoulou A., Rodríguez J. M., Galindo A., Peñalva M. A. Long-distance movement of Aspergillus nidulans early endosomes on microtubule tracks. Traffic. 2009;10:57–75. - PubMed
-
- Apostolaki A., Erpapazoglou Z., Harispe L., Billini M., Kafasla P., Kizis D., Peñalva M. A., Scazzocchio C., Sophianopoulou V. AgtA, the dicarboxylic amino acid transporter of Aspergillus nidulans, is concertedly down-regulated by exquisite sensitivity to nitrogen metabolite repression and ammonium-elicited endocytosis. Eukaryot. Cell. 2009;8:339–352. - PMC - PubMed
-
- Bilodeau P. S., Urbanowski J. L., Winistorfer S. C., Piper R. C. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol. 2002;4:534–539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

