The retinoblastoma protein modulates Tbx2 functional specificity
- PMID: 20534814
- PMCID: PMC2912361
- DOI: 10.1091/mbc.E09-12-1029
The retinoblastoma protein modulates Tbx2 functional specificity
Abstract
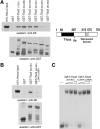
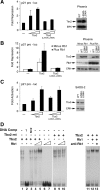
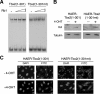
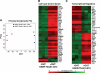
Tbx2 is a member of a large family of transcription factors defined by homology to the T-box DNA-binding domain. Tbx2 plays a key role in embryonic development, and in cancer through its capacity to suppress senescence and promote invasiveness. Despite its importance, little is known of how Tbx2 is regulated or how it achieves target gene specificity. Here we show that Tbx2 specifically associates with active hypophosphorylated retinoblastoma protein (Rb1), a known regulator of many transcription factors involved in cell cycle progression and cellular differentiation, but not with the Rb1-related proteins p107 or p130. The interaction with Rb1 maps to a domain immediately carboxy-terminal to the T-box and enhances Tbx2 DNA binding and transcriptional repression. Microarray analysis of melanoma cells expressing inducible dominant-negative Tbx2, comprising the T-box and either an intact or mutated Rb1 interaction domain, shows that Tbx2 regulates the expression of many genes involved in cell cycle control and that a mutation which disrupts the Rb1-Tbx2 interaction also affects Tbx2 target gene selectivity. Taken together, the data show that Rb1 is an important determinant of Tbx2 functional specificity.
Figures

References
-
- Abrahams A., Mowla S., Parker M. I., Goding C. R., Prince S. UV-mediated regulation of the anti-senescence factor Tbx2. J. Biol. Chem. 2008;283:2223–2230. - PubMed
-
- Amellem O., Stokke T., Sandvik J. A., Pettersen E. O. The retinoblastoma gene product is reversibly dephosphorylated and bound in the nucleus in S and G2 phases during hypoxic stress. Exp. Cell Res. 1996;227:106–115. - PubMed
-
- Bandyopadhyay D., Medrano E. E. Melanin accumulation accelerates melanocyte senescence by a mechanism involving p16INK4a/CDK4/pRB and E2F1. Ann. NY Acad. Sci. 2000;908:71–84. - PubMed
-
- Bilican B., Goding C. R. Cell cycle regulation of the T-box transcription factor tbx2. Exp. Cell Res. 2006;312:2358–2366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

