Role of chondroitin sulfate proteoglycans in axonal conduction in Mammalian spinal cord
- PMID: 20534825
- PMCID: PMC3531897
- DOI: 10.1523/JNEUROSCI.4659-09.2010
Role of chondroitin sulfate proteoglycans in axonal conduction in Mammalian spinal cord
Abstract
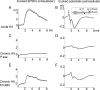
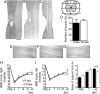
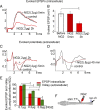
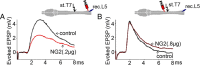
Chronic unilateral hemisection (HX) of the adult rat spinal cord diminishes conduction through intact fibers in the ventrolateral funiculus (VLF) contralateral to HX. This is associated with a partial loss of myelination from fibers in the VLF (Arvanian et al., 2009). Here, we again measured conduction through the VLF using electrical stimulation while recording the resulting volley and synaptic potentials in target motoneurons. We found that intraspinal injection of chondroitinase-ABC, known to digest chondroitin sulfate proteoglycans (CSPGs), prevented the decline of axonal conduction through intact VLF fibers across from chronic T10 HX. Chondroitinase treatment was also associated with behavior suggestive of an improvement of locomotor function after chronic HX. To further study the role of CSPGs in axonal conduction, we injected three purified CSPGs, NG2 and neurocan, which increase in the vicinity of a spinal injury, and aggrecan, which decreases, into the lateral column of the uninjured cord at T10 in separate experiments. Intraspinal injection of NG2 acutely depressed axonal conduction through the injected region in a dose-dependent manner. Similar injections of saline, aggrecan, or neurocan had no significant effect. Immunofluorescence staining experiments revealed the presence of endogenous and exogenous NG2 at some nodes of Ranvier. These results identify a novel acute action of CSPGs on axonal conduction in the spinal cord and suggest that antagonism of proteoglycans reverses or prevents the decline of axonal conduction, in addition to stimulating axonal growth.
Figures
References
-
- Arvanian VL, Mendell LM. Removal of NMDA receptor Mg2+ block extends the action of NT-3 on synaptic transmission in neonatal rat motoneurons. J Neurophys. 2001;86:123–129. - PubMed
-
- Basso MD, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
-
- Bekku Y, Rauch U, Ninomiya Y, Oohashi T. Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. J Neurochem. 2009;108:1266–1276. - PubMed
-
- Black JA, Waxman SG, Smith KJ. Remyelination of dorsal column axons by endogenous Schwann cells restores the normal pattern of Nav1.6 and Kv1.2 at nodes of Ranvier. Brain. 2006;129:1319–1329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
