Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration
- PMID: 20534829
- PMCID: PMC6632683
- DOI: 10.1523/JNEUROSCI.0372-10.2010
Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration
Abstract
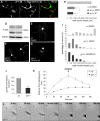
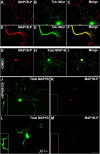
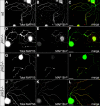
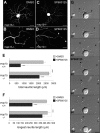
c-Jun N-terminal kinases (JNKs) (comprising JNK1-3 isoforms) are members of the MAPK (mitogen-activated protein kinase) family, activated in response to various stimuli including growth factors and inflammatory cytokines. Their activation is facilitated by scaffold proteins, notably JNK-interacting protein-1 (JIP1). Originally considered to be mediators of neuronal degeneration in response to stress and injury, recent studies support a role of JNKs in early stages of neurite outgrowth, including adult axonal regeneration. However, the function of individual JNK isoforms, and their potential effector molecules, remained unknown. Here, we analyzed the role of JNK signaling during axonal regeneration from adult mouse dorsal root ganglion (DRG) neurons, combining pharmacological JNK inhibition and mice deficient for each JNK isoform and for JIP1. We demonstrate that neuritogenesis is delayed by lack of JNK2 and JNK3, but not JNK1. JNK signaling is further required for sustained neurite elongation, as pharmacological JNK inhibition resulted in massive neurite retraction. This function relies on JNK1 and JNK2. Neurite regeneration of jip1(-/-) DRG neurons is affected at both initiation and extension stages. Interestingly, activated JNKs (phospho-JNKs), as well as JIP1, are also present in the cytoplasm of sprouting or regenerating axons, suggesting a local action on cytoskeleton proteins. Indeed, we have shown that JNK1 and JNK2 regulate the phosphorylation state of microtubule-associated protein MAP1B, whose role in axonal regeneration was previously characterized. Moreover, lack of MAP1B prevents neurite retraction induced by JNK inhibition. Thus, signaling by individual JNKs is differentially implicated in the reorganization of the cytoskeleton, and neurite regeneration.
Figures

References
-
- Ahmad FJ, Hughey J, Wittmann T, Hyman A, Greaser M, Baas PW. Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nat Cell Biol. 2000;2:276–280. - PubMed
-
- Alfei L, Soares S, Alunni A, Ravaille-Veron M, Von Boxberg Y, Nothias F. Expression of MAP1B protein and its phosphorylated form MAP1B-P in the CNS of a continuously growing fish, the rainbow trout. Brain Res. 2004;1009:54–66. - PubMed
-
- Baas PW, Ahmad FJ. Force generation by cytoskeletal motor proteins as a regulator of axonal elongation and retraction. Trends Cell Biol. 2001;11:244–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
