Rab11 supports amphetamine-stimulated norepinephrine transporter trafficking
- PMID: 20534835
- PMCID: PMC2935280
- DOI: 10.1523/JNEUROSCI.4574-09.2010
Rab11 supports amphetamine-stimulated norepinephrine transporter trafficking
Abstract
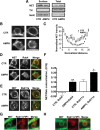
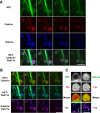
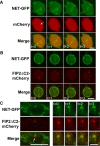
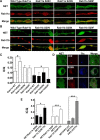
The norepinephrine transporter (NET) is a presynaptic plasma membrane protein that mediates reuptake of synaptically released norepinephrine. NET is also a major target for medications used for the treatment of depression, attention deficit/hyperactivity disorder, narcolepsy, and obesity. NET is regulated by numerous mechanisms, including catalytic activation and membrane trafficking. Amphetamine (AMPH), a psychostimulant and NET substrate, has also been shown to induce NET trafficking. However, neither the molecular basis nor the nature of the relevant membrane compartments of AMPH-modulated NET trafficking has been defined. Indeed, direct visualization of drug-modulated NET trafficking in neurons has yet to be demonstrated. In this study, we used a recently developed NET antibody and the presence of large presynaptic boutons in sympathetic neurons to examine basal and AMPH-modulated NET trafficking. Specifically, we establish a role for Rab11 in AMPH-induced NET trafficking. First, we found that, in cortical slices, AMPH induces a reduction in surface NET. Next, we observed AMPH-induced accumulation and colocalization of NET with Rab11a and Rab4 in presynaptic boutons of cultured neurons. Using tagged proteins, we demonstrated that NET and a truncated Rab11 effector (FIP2DeltaC2) do not redistribute in synchrony, whereas NET and wild-type Rab11a do. Analysis of various Rab11a/b mutants further demonstrates that Rab11 regulates NET trafficking. Expression of the truncated Rab11a effector (FIP2DeltaC2) attenuates endogenous Rab11 function and prevented AMPH-induced NET internalization as does GDP-locked Rab4 S22N. Our data demonstrate that AMPH leads to an increase of NET in endosomes of single boutons and varicosities in a Rab11-dependent manner.
Figures




References
-
- Ahmed BA, Jeffus BC, Bukhari SI, Harney JT, Unal R, Lupashin VV, van der Sluijs P, Kilic F. Serotonin transamidates Rab4 and facilitates its binding to the C terminus of serotonin transporter. J Biol Chem. 2008;283:9388–9398. - PubMed
-
- Apparsundaram S, Galli A, DeFelice LJ, Hartzell HC, Blakely RD. Acute regulation of norepinephrine transport: I. Protein kinase C-linked muscarinic receptors influence transport capacity and transporter density in SK-N-SH cells. J Pharmacol Exp Ther. 1998a;287:733–743. - PubMed
-
- Apparsundaram S, Schroeter S, Giovanetti E, Blakely RD. Acute regulation of norepinephrine transport: II. PKC-modulated surface expression of human norepinephrine transporter proteins. J Pharmacol Exp Ther. 1998b;287:744–751. - PubMed
-
- Becker KP, Hannun YA. cPKC-dependent sequestration of membrane-recycling components in a subset of recycling endosomes. J Biol Chem. 2003;278:52747–52754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
