Ionic mechanisms underlying inflammatory mediator-induced sensitization of dural afferents
- PMID: 20534836
- PMCID: PMC2900838
- DOI: 10.1523/JNEUROSCI.6053-09.2010
Ionic mechanisms underlying inflammatory mediator-induced sensitization of dural afferents
Abstract
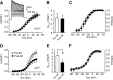
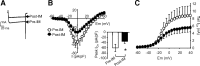
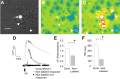
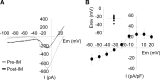
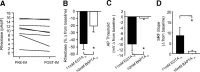
Migraineurs experience debilitating headaches that result from neurogenic inflammation of the dura and subsequent sensitization of dural afferents. Given the importance of inflammatory mediator (IM)-induced dural afferent sensitization to this pain syndrome, the present study was designed to identify ionic mechanisms underlying this process. Trigeminal ganglion neurons from adult female Sprague Dawley rats were acutely dissociated 10-14 d after application of retrograde tracer DiI onto the dura. Modulation of ion channels and changes in excitability were measured in the absence and presence of IMs (in mum: 1 prostaglandin, 10 bradykinin, and 1 histamine) using whole-cell and perforated-patch recordings. Fura-2 was used to assess changes in intracellular Ca(2+). IMs modulated a number of currents in dural afferents, including those both expected and/or previously described [i.e., an increase in tetrodotoxin-resistant voltage-gated Na(+) current (TTX-R I(Na)) and a decrease in voltage-gated Ca(2+) current] as well currents never before described in sensory neurons (i.e., a decrease in a Ca(2+)-dependent K(+) current and an increase in a Cl(-) current), and produced a sustained elevation in intracellular Ca(2+). Although several of these currents, in particular TTX-R I(Na), appear to contribute to the sensitization of dural afferents, the Cl(-) current is the primary mechanism underlying this process. Activation of this current plays a dominant role in the sensitization of dural afferents because of the combination of the density and biophysical properties of TTX-R I(Na), and the high level of intracellular Cl(-) in these neurons. These results suggest novel targets for the development of antimigraine agents.
Figures




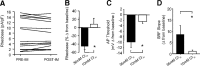
References
-
- Akaike N. Gramicidin perforated patch recording and intracellular chloride activity in excitable cells. Prog Biophys Mol Biol. 1996;65:251–264. - PubMed
-
- André S, Boukhaddaoui H, Campo B, Al-Jumaily M, Mayeux V, Greuet D, Valmier J, Scamps F. Axotomy-induced expression of calcium-activated chloride current in subpopulations of mouse dorsal root ganglion neurons. J Neurophysiol. 2003;90:3764–3773. - PubMed
-
- Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol. 2004;287:G845–G855. - PubMed
-
- Bolay H, Moskowitz MA. Mechanisms of pain modulation in chronic syndromes. Neurology. 2002;59:S2–S7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
