Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells
- PMID: 20534844
- PMCID: PMC6632678
- DOI: 10.1523/JNEUROSCI.6170-09.2010
Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells
Abstract
The limited generation of neurons during adulthood is controlled by a balance between quiescence and recruitment of neural stem cells (NSCs). We use here the germinal zone of the zebrafish adult telencephalon to examine how the frequency of NSC divisions is regulated. We show, using several in vivo techniques, that progenitors transit back and forth between the quiescent and dividing state, according to varying levels of Notch activity: Notch induction drives progenitors into quiescence, whereas blocking Notch massively reinitiates NSC division and subsequent commitment toward becoming neurons. Notch activation appears predominantly triggered by newly recruited progenitors onto their neighbors, suggesting an involvement of Notch in a self-limiting mechanism, once neurogenesis is started. These results identify for the first time a lateral inhibition-like mechanism in the context of adult neurogenesis and suggest that the equilibrium between quiescence and neurogenesis in the adult brain is controlled by fluctuations of Notch activity, thereby regulating the amount of adult-born neurons.
Figures
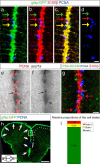



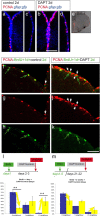
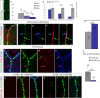
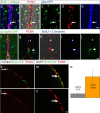

References
-
- Adolf B, Chapouton P, Lam CS, Topp S, Tannhäuser B, Strähle U, Götz M, Bally-Cuif L. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol. 2006;295:278–293. - PubMed
-
- Aizawa H, Goto M, Sato T, Okamoto H. Temporally regulated asymmetric neurogenesis causes left-right difference in the zebrafish habenular structures. Dev Cell. 2007;12:87–98. - PubMed
-
- Baek JH, Hatakeyama J, Sakamoto S, Ohtsuka T, Kageyama R. Persistent and high levels of Hes1 expression regulate boundary formation in the developing central nervous system. Development. 2006;133:2467–2476. - PubMed
-
- Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expr Patterns. 2006;6:1007–1013. - PubMed
-
- Bernardos RL, Lentz SI, Wolfe MS, Raymond PA. Notch-Delta signaling is required for spatial patterning and Muller glia differentiation in the zebrafish retina. Dev Biol. 2005;278:381–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
