Selective induction of host genes by MVA-B, a candidate vaccine against HIV/AIDS
- PMID: 20534857
- PMCID: PMC2916545
- DOI: 10.1128/JVI.00749-10
Selective induction of host genes by MVA-B, a candidate vaccine against HIV/AIDS
Abstract
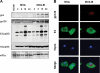
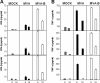
The aim of this study was to define the effects on antigen-presenting cells of the expression of HIV antigens from an attenuated poxvirus vector. We have analyzed the transcriptional changes in gene expression following infection of human immature monocyte-derived dendritic cells (DC) with recombinant modified vaccinia virus Ankara (MVA) expressing the genes encoding the gp120 and Gag-Pol-Nef antigens of HIV type 1 clade B (referred to as MVA-B) versus parental MVA infection. Using microarray technology and real-time reverse transcription-PCR, we demonstrated that the HIV proteins induced the expression of cytokines, cytokine receptors, chemokines, chemokine receptors, and molecules involved in antigen uptake and processing, including major histocompatibility complex (MHC) genes. Levels of mRNAs for interleukin-1, beta interferon, CCR8, and SCYA20 were higher after HIV antigen production. MVA-B infection also modulated the expression of antigen processing and presentation genes: the gene for MICA was upregulated, whereas those for HLA-DRA and HSPA5 were downregulated. Indeed, the increased expression of the gene for MICA, a glycoprotein related to major histocompatibility complex class I molecules, was shown to enhance the interaction between MVA-B-infected target cells and cytotoxic lymphocytes. The expression profiles of the genes for protein kinases such as JAK1 and IRAK2 were activated after HIV antigen expression. Several genes included in the JAK-STAT and mitogen-activated protein kinase signaling pathways were regulated after HIV antigen expression. Our findings provide the first gene signatures in DC of a candidate MVA-B vaccine expressing four HIV antigens and identified the biological roles of some of the regulatory genes, like that for MICA, which will help in the design of more effective MVA-derived vaccines.
Figures



References
-
- Albert, M. L., M. Jegathesan, and R. B. Darnell. 2001. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat. Immunol. 2:1010-1017. - PubMed
-
- Alcamí, A., J. A. Symons, P. D. Collins, T. J. Williams, and G. L. Smith. 1998. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J. Immunol. 160:624-633. - PubMed
-
- Reference deleted.
-
- Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365-396. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

