Neutralization of human respiratory syncytial virus infectivity by antibodies and low-molecular-weight compounds targeted against the fusion glycoprotein
- PMID: 20534864
- PMCID: PMC2916552
- DOI: 10.1128/JVI.00447-10
Neutralization of human respiratory syncytial virus infectivity by antibodies and low-molecular-weight compounds targeted against the fusion glycoprotein
Abstract
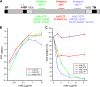
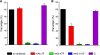
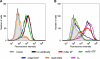
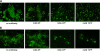
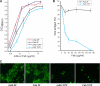
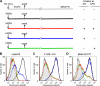
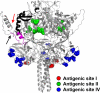
Human respiratory syncytial virus (HRSV) fusion (F) protein is an essential component of the virus envelope that mediates fusion of the viral and cell membranes, and, therefore, it is an attractive target for drug and vaccine development. Our aim was to analyze the neutralizing mechanism of anti-F antibodies in comparison with other low-molecular-weight compounds targeted against the F molecule. It was found that neutralization by anti-F antibodies is related to epitope specificity. Thus, neutralizing and nonneutralizing antibodies could bind equally well to virions and remained bound after ultracentrifugation of the virus, but only the former inhibited virus infectivity. Neutralization by antibodies correlated with inhibition of cell-cell fusion in a syncytium formation assay, but not with inhibition of virus binding to cells. In contrast, a peptide (residues 478 to 516 of F protein [F478-516]) derived from the F protein heptad repeat B (HRB) or the organic compound BMS-433771 did not interfere with virus infectivity if incubated with virus before ultracentrifugation or during adsorption of virus to cells at 4 degrees C. These inhibitors must be present during virus entry to effect HRSV neutralization. These results are best interpreted by asserting that neutralizing antibodies bind to the F protein in virions interfering with its activation for fusion. Binding of nonneutralizing antibodies is not enough to block this step. In contrast, the peptide F478-516 or BMS-433771 must bind to F protein intermediates generated during virus-cell membrane fusion, blocking further development of this process.
Figures



References
-
- Andries, K., M. Moeremans, T. Gevers, R. Willebrords, C. Sommen, J. Lacrampe, F. Janssens, and P. R. Wyde. 2003. Substituted benzimidazoles with nanomolar activity against respiratory syncytial virus. Antiviral Res. 60:209-219. - PubMed
-
- Arbiza, J., G. Taylor, J. A. Lopez, J. Furze, S. Wyld, P. Whyte, E. J. Stott, G. Wertz, W. Sullender, M. Trudel, et al. 1992. Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial virus. J. Gen. Virol. 73:2225-2234. - PubMed
-
- Baker, K. A., R. E. Dutch, R. A. Lamb, and T. S. Jardetzky. 1999. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell 3:309-319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

