Short-term fasting induces profound neuronal autophagy
- PMID: 20534972
- PMCID: PMC3106288
- DOI: 10.4161/auto.6.6.12376
Short-term fasting induces profound neuronal autophagy
Abstract
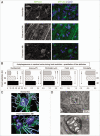
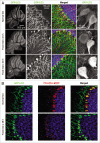
Disruption of autophagy--a key homeostatic process in which cytosolic components are degraded and recycled through lysosomes--can cause neurodegeneration in tissue culture and in vivo. Upregulation of this pathway may be neuroprotective, and much effort is being invested in developing drugs that cross the blood brain barrier and increase neuronal autophagy. One well-recognized way of inducing autophagy is by food restriction, which upregulates autophagy in many organs including the liver; but current dogma holds that the brain escapes this effect, perhaps because it is a metabolically privileged site. Here, we have re-evaluated this tenet using a novel approach that allows us to detect, enumerate and characterize autophagosomes in vivo. We first validate the approach by showing that it allows the identification and characterization of autophagosomes in the livers of food-restricted mice. We use the method to identify constitutive autophagosomes in cortical neurons and Purkinje cells, and we show that short-term fasting leads to a dramatic upregulation in neuronal autophagy. The increased neuronal autophagy is revealed by changes in autophagosome abundance and characteristics, and by diminished neuronal mTOR activity in vivo, demonstrated by a reduction in levels of phosphorylated S6 ribosomal protein in Purkinje cells. The increased abundance of autophagosomes in Purkinje cells was confirmed using transmission electron microscopy. Our data lead us to speculate that sporadic fasting might represent a simple, safe and inexpensive means to promote this potentially therapeutic neuronal response.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- AI-042314/AI/NIAID NIH HHS/United States
- T32 NS41219/NS/NINDS NIH HHS/United States
- R01 AI027028/AI/NIAID NIH HHS/United States
- R21 AI027028/AI/NIAID NIH HHS/United States
- R01 AI042314/AI/NIAID NIH HHS/United States
- R01 AI077607/AI/NIAID NIH HHS/United States
- T32 NS041219/NS/NINDS NIH HHS/United States
- R01 HL093177/HL/NHLBI NIH HHS/United States
- F32 AI078660/AI/NIAID NIH HHS/United States
- R56 AI027028/AI/NIAID NIH HHS/United States
- AI-077607/AI/NIAID NIH HHS/United States
- AI-027028/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
