Oxidative stress promotes myofibroblast differentiation and tumour spreading
- PMID: 20535745
- PMCID: PMC3377319
- DOI: 10.1002/emmm.201000073
Oxidative stress promotes myofibroblast differentiation and tumour spreading
Abstract
JunD regulates genes involved in antioxidant defence. We took advantage of the chronic oxidative stress resulting from junD deletion to examine the role of reactive oxygen species (ROS) in tumour development. In a model of mammary carcinogenesis, junD inactivation increased tumour incidence and revealed an associated reactive stroma. junD-inactivation in the stroma was sufficient to shorten tumour-free survival rate and enhance metastatic spread. ROS promoted conversion of fibroblasts into highly migrating myofibroblasts through accumulation of the hypoxia-inducible factor (HIF)-1alpha transcription factor and the CXCL12 chemokine. Accordingly, treatment with an antioxidant reduced the levels of HIF and CXCL12 and numerous myofibroblast features. CXCL12 accumulated in the stroma of HER2-human breast adenocarcinomas. Moreover, HER2 tumours exhibited a high proportion of myofibroblasts, which was significantly correlated to nodal metastases. Interestingly, this subset of tumours exhibited a significant nuclear exclusion of JunD and revealed an associated oxido-reduction signature, further demonstrating the relevance of our findings in human cancers. Collectively, our data uncover a new mechanism by which oxidative stress increases the migratory properties of stromal fibroblasts, which in turn potentiate tumour dissemination.
Figures
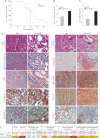
Kaplan–Meyer tumour-free survival curve of ras junD+/+ (referred to as ras) animals (n = 12) and ras junD−/−mice (n = 12) (p = 0.0092, log-rank test).
Number of tumours per animal in ras (n = 12) and ras junD−/− (n = 12) mice.
Tumour volumes in ras (n = 10) and ras junD−/− (n = 9) mice.
Sections and histological analysis of epithelial tumours (a–h) and immediate adjacent stroma (i–t) from ras or ras junD−/− animals. Sections have been coloured with HES (haematoxylin-eosin-saffranin) (a,b,k,l), Masson's trichrome (c,d) or immunostained with specific antibodies, as indicated (e–j,m–t).
Percentage of epithelial and fibroblastic compartments in the tumours has been evaluated using E-cadherin and Vimentin-specific staining, respectively. Are also indicated intensity (Int), percentage of positive cells (%) and H scoring (Int × %) for E-cadherin, Vimentin and SM-α-actin-staining as well as the number of F4/80- or CD45-positive cells per µm2. n represents the number of animals analysed per genotype; n represents the number of tumours analysed per genotype. Data are means ± SEM. *p < 0.05 by student's test. Scale bars = 20 µm in (Da,b,k,l) and 40 µm in (Dc–j,Dm–t).

A. Kaplan–Meyer tumour-free survival curve of wt (n = 10) and junD−/− mice (n = 10) in graft experiments using B16F10 cells (p = 0.041, log-rank test).
B, C. Representative immunohistochemistry of tumours from injected wt (a,c) and junD−/− mice (b,d) using SM-α-actin and CXCL12-specific antibodies.
D. Typical HES views of lungs from injected mice. Sections show large (b) and medium (d) sizes of metastatic nodules in junD−/− mice compared to wt animals (a,c).
E. Number of total, small-sized (<10 cells), medium-sized (10 cells < × < 50 cells) and large-sized (>50 cells) metastasis in wt and junD−/− mice. Numbers below indicate the percentage of large-, medium- and small-sized metastasis in the respective populations.
F. Tumour volumes in junD−/− mice treated daily either with control siRNA (black) or with specific CXCL12-directed siRNA. Data are means ± SEM. *p < 0.05 and ***p < 0.005 by student's test. N represents the number of tumours analysed per genotype. Scale bars = 40 µm.

Venn's diagram showing the number of common up-regulated genes in CAF (from Allinen's and Farmer's studies) and junD−/− fibroblasts. On the right panel, the 14 genes common to the three lists are listed.
Representative immunofluorescence staining from wt and junD−/− cells using specific antibodies, as indicated. Arrows indicate typical staining. Inserted section in (f) shows a higher magnification (100×) image of a representative AJ co-stained with SM-α-actin (in green) and N-cadherin (in red).
Western blots of whole cell extracts from wt and junD−/− fibroblasts. Ponceau colouration was used as an internal control for each protein loading; a representative gel is shown.
Migration assay of wt compare with junD−/− fibroblasts. *p < 0.05 by student test. Scale bars = 10 µm (Ba–d,g–l) and 5 µm in (Be,f).
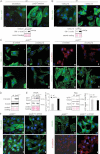
SM-α-actin staining in wt fibroblasts after incubation in wt or junD−/−-conditioned medium. At the bottom is the corresponding Western blot.
SM-α-actin staining in wt fibroblasts after addition of exogenous CXCL12 protein. At the bottom is the corresponding Western blot.
Representative immunofluorescence of myofibroblast markers in junD−/− fibroblasts after transfection with a scramble siRNA (si control) (a,c,e,g) or with a CXCL12-directed siRNA (si CXCL12) (b,d,f,h).
Western blots from wt and junD−/− whole cell extracts showing p44 and p42 MAPK (Erk1/2) and their phosphorylated forms.
Representative GST pull-down assays on wt and junD−/− fibroblasts for RhoA (left panel) and Rac (right panel). GTP-bound form and total amount (input) of each protein are shown. Histograms show relative Rho- or Rac-GTP levels normalized to their respective total protein amounts.
Representative immunofluorescence of myofibroblast markers in junD−/− fibroblasts either untreated (a,c,e,g) or incubated with exoenzyme C3 transferase (b,d,f,h). Scale bars = 10 µm.

Representative immunofluorescence of myofibroblast markers in junD−/− fibroblasts after transfection with a scramble siRNA (si control) (a,c,e,g) or with an HIF-1α-directed siRNA (si HIF) (b,d,f,h).
Representative immunofluorescence of myofibroblast markers in wt fibroblasts either untreated (a,c,e,g) or incubated with DFO (b,d,f,h).
Representative GST pull-down assays for RhoA (left panel) and Rac (right panel) on wt fibroblasts with or without DFO.
Representative immunofluorescence of myofibroblast markers in junD−/− fibroblasts either untreated (a,c,e,g) or incubated with NAC (b,d,f,h). Scale bars = 10 µm.

Sections and histological analysis of HER2 (a–c), BLC (d.f) and Lum-A (g–i) human breast tumours using CXCL12-specific antibody.
Sections and histological analysis of HER2 (a–c), BLC (d.f) and Lum-A (g–i) human breast tumours using CXCL4-specific antibody. Scale bars = 40 µm.

Representative staining of SM-α-actin in HER2 (a–c), BLC (d–f) and Lum-A (g–i) human breast tumours. Arrowheads indicate SM-α-actin staining in epithelial cells in BLC (f). p-values are as in Fig 6.
Representative graph of the percentage of fibroblasts compared to total cells forming the tumour in each breast cancer subtype. p-values by χ2 test are highly significant between BLC and HER2 or Lum-A (≤0.001) and non-significant between HER2 and Lum-A.
Gene Ontology pathways significantly at least 2-fold up-regulated in HER2 versus Lum-A, BLC versus Lum-A and HER2 versus BLC, as indicated. p-values by Fisher Exact test adjusted using the Benjamini–Hochberg correction are indicated.

Representative staining of JunD in HER2 (a–c), BLC (d–f) and Lum-A (g–i) human breast tumours. p-values by χ2 test are highly significant between BLC and HER2 or Lum-A.
Model: In carcinoma, chronic oxidative stress promotes the conversion of fibroblasts into myofibroblasts, contractile cells with high migration properties capacity. Pro-invasive myofibroblast properties result from ROS-mediated accumulation of the pro-angiogenic HIF-1α factor and the CXCL12 chemokine. The origin of oxidative stress can be either intrinsic or extrinsic to fibroblasts. Indeed, fibroblasts may acquire genetic alteration, such as junD inactivation, which will intrinsically increase ROS contents. Since genetic alteration in stromal fibroblasts may be rare events, stress may also more often originate from tumour cells themselves. Accordingly, we identified a signature characteristic of stress–response in HER2-amplified tumours. In this set of tumours, stress can originate from non-exclusive mechanisms, such as JunD nuclear exclusion, up-regulation of Nox4 or HER2/ERBB2 amplification per se, since it has been previously demonstrated that growth factor-stimulated RTKs enhance H2O2 levels through activation of Nox. H2O2 is highly diffusible and can easily cross-cellular membranes to act on surrounding fibroblasts. Acute stress in fibroblasts increases levels of HIF and CXCL12 that, in turn, convert fibroblasts into myofibroblasts. These highly contractile SM-α-actin-expressing cells would subsequently promote migration and dissemination of neoplastic cells.
References
-
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. - PubMed
-
- Aslan M, Ozben T. Oxidants in receptor tyrosine kinase signal transduction pathways. Antioxid Redox Signal. 2003;5:781–788. - PubMed
-
- Bartlett JM, Ellis IO, Dowsett M, Mallon EA, Cameron DA, Johnston S, Hall E, A'Hern R, Peckitt C, Bliss JM, et al. Human epidermal growth factor receptor 2 status correlates with lymph node involvement in patients with estrogen receptor (ER) negative, but with grade in those with ER-positive early-stage breast cancer suitable for cytotoxic chemotherapy. J Clin Oncol. 2007;25:4423–4430. - PubMed
-
- Bartolome RA, Galvez BG, Longo N, Baleux F, Van Muijen GN, Sanchez-Mateos P, Arroyo AG, Teixido J. Stromal cell-derived factor-1alpha promotes melanoma cell invasion across basement membranes involving stimulation of membrane-type 1 matrix metalloproteinase and Rho GTPase activities. Cancer Res. 2004;64:2534–2543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

