Role of zinc in human islet amyloid polypeptide aggregation
- PMID: 20536124
- PMCID: PMC2904811
- DOI: 10.1021/ja1007867
Role of zinc in human islet amyloid polypeptide aggregation
Abstract
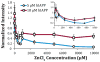
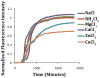
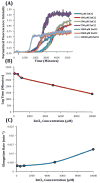
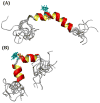
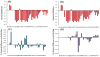
Human Islet Amyloid Polypeptide (hIAPP) is a highly amyloidogenic protein found in islet cells of patients with type II diabetes. Because hIAPP is highly toxic to beta-cells under certain conditions, it has been proposed that hIAPP is linked to the loss of beta-cells and insulin secretion in type II diabetics. One of the interesting questions surrounding this peptide is how the toxic and aggregation prone hIAPP peptide can be maintained in a safe state at the high concentrations that are found in the secretory granule where it is stored. We show here zinc, which is found at millimolar concentrations in the secretory granule, significantly inhibits hIAPP amyloid fibrillogenesis at concentrations similar to those found in the extracellular environment. Zinc has a dual effect on hIAPP fibrillogenesis: it increases the lag-time for fiber formation and decreases the rate of addition of hIAPP to existing fibers at lower concentrations, while having the opposite effect at higher concentrations. Experiments at an acidic pH which partially neutralizes the change in charge upon zinc binding show inhibition is largely due to an electrostatic effect at His18. High-resolution structures of hIAPP determined from NMR experiments confirm zinc binding to His18 and indicate zinc induces localized disruption of the secondary structure of IAPP in the vicinity of His18 of a putative helical intermediate of IAPP. The inhibition of the formation of aggregated and toxic forms of hIAPP by zinc provides a possible mechanism between the recent discovery of linkage between deleterious mutations in the SLC30A8 zinc transporter, which transports zinc into the secretory granule, and type II diabetes.
Figures
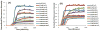




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

