Conformational stability of Syrian hamster prion protein PrP(90-231)
- PMID: 20536231
- PMCID: PMC2902166
- DOI: 10.1021/ja100243h
Conformational stability of Syrian hamster prion protein PrP(90-231)
Abstract
Many transmissible spongiform encephalopathies (TSEs) are believed to be caused by a misfolded form of the normal cellular prion protein (PrP(C)) known as PrP(Sc). While PrP(Sc) is known to be exceptionally stable and resistant to protease degradation, PrP(C) has not shown these same unusual characteristics. However, using ion mobility spectrometry mass spectrometry (IMS-MS), we found evidence for at least one very stable conformation of a truncated form of recombinant PrP(C) consisting of residues 90-231, which resists unfolding in the absence of solvent at high injection energies and at temperatures in excess of 600 K. We also report the first absolute collision cross sections measured for recombinant Syrian hamster prion protein PrP(90-231).
Figures


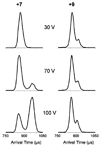

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

