Cytochrome C encapsulating theranostic nanoparticles: a novel bifunctional system for targeted delivery of therapeutic membrane-impermeable proteins to tumors and imaging of cancer therapy
- PMID: 20536259
- PMCID: PMC2914151
- DOI: 10.1021/mp100043h
Cytochrome C encapsulating theranostic nanoparticles: a novel bifunctional system for targeted delivery of therapeutic membrane-impermeable proteins to tumors and imaging of cancer therapy
Abstract
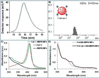
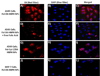
The effective administration of therapeutic proteins has received increased attention for the treatment of various diseases. Encapsulation of these proteins in various matrices, as a method of protein structure and function preservation, is a widely used approach that results in maintenance of the protein's function. However, targeted delivery and tracking of encapsulated therapeutic proteins to the affected cells is still a challenge. In an effort to advance the targeted delivery of a functional apoptosis-initiating protein (cytochrome c) to cancer cells, we formulated theranostic polymeric nanoparticles for the simultaneous encapsulation of cytochrome c and a near-infrared dye to folate-expressing cancer cells. The polymeric nanoparticles were prepared using a novel water-soluble hyperbranched polyhydroxyl polymer that allows for dual encapsulation of a hydrophilic protein and an amphiphilic fluorescent dye. Our protein therapeutic cargo is the endogenous protein cytochrome c, which upon cytoplasmic release, initiates an apoptotic response leading to programmed cell death. Results indicate that encapsulation of cytochrome c within the nanoparticle's cavities preserved the protein's enzymatic activity. The potential therapeutic property of these nanoparticles was demonstrated by the induction of apoptosis upon intracellular delivery. Furthermore, targeted delivery of cytochrome c to folate-receptor-positive cancer cells was achieved via conjugation of folic acid to the nanoparticle's surface, whereas the nanoparticle's theranostic properties were conferred via the coencapsulation of cytochrome c and a fluorescent dye. Considering that these theranostic nanoparticles can carry an endogenous cellular apoptotic initiator (cytochrome c) and a fluorescent tag (ICG) commonly used in the clinic, their use and potential translation into the clinic is anticipated, facilitating the monitoring of tumor regression.
Figures










Similar articles
-
Doxorubicin-loaded protease-activated near-infrared fluorescent polymeric nanoparticles for imaging and therapy of cancer.Int J Nanomedicine. 2018 Oct 31;13:6961-6986. doi: 10.2147/IJN.S174068. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30464453 Free PMC article.
-
Activation of caspase-dependent apoptosis by intracellular delivery of Cytochrome c-based nanoparticles.J Nanobiotechnology. 2014 Sep 2;12:33. doi: 10.1186/s12951-014-0033-9. J Nanobiotechnology. 2014. PMID: 25179308 Free PMC article.
-
Folate-receptor-targeted laser-activable poly(lactide-co-glycolic acid) nanoparticles loaded with paclitaxel/indocyanine green for photoacoustic/ultrasound imaging and chemo/photothermal therapy.Int J Nanomedicine. 2018 Sep 6;13:5139-5158. doi: 10.2147/IJN.S167043. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30233177 Free PMC article.
-
Folic acid-indocyanine green-poly(d,l-lactide-coglycolide)-lipid nanoparticles.2012 Aug 30 [updated 2012 Sep 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Aug 30 [updated 2012 Sep 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23035307 Free Books & Documents. Review.
-
Nanodrug delivery systems: a promising technology for detection, diagnosis, and treatment of cancer.AAPS PharmSciTech. 2014 Jun;15(3):709-21. doi: 10.1208/s12249-014-0089-8. Epub 2014 Feb 19. AAPS PharmSciTech. 2014. PMID: 24550101 Free PMC article. Review.
Cited by
-
Magnetite Nanoparticles in Magnetic Hyperthermia and Cancer Therapies: Challenges and Perspectives.Nanomaterials (Basel). 2022 May 25;12(11):1807. doi: 10.3390/nano12111807. Nanomaterials (Basel). 2022. PMID: 35683663 Free PMC article. Review.
-
Chemical Modification of Cytochrome C for Acid-Responsive Intracellular Apoptotic Protein Delivery for Cancer Eradication.Pharmaceutics. 2024 Jan 4;16(1):71. doi: 10.3390/pharmaceutics16010071. Pharmaceutics. 2024. PMID: 38258082 Free PMC article.
-
Optimization and Characterization of Protein Nanoparticles for the Targeted and Smart Delivery of Cytochrome c to Non-Small Cell Lung Carcinoma.Cancers (Basel). 2020 May 13;12(5):1215. doi: 10.3390/cancers12051215. Cancers (Basel). 2020. PMID: 32413975 Free PMC article.
-
Membrane-Interacting DNA Nanotubes Induce Cancer Cell Death.Nanomaterials (Basel). 2021 Aug 4;11(8):2003. doi: 10.3390/nano11082003. Nanomaterials (Basel). 2021. PMID: 34443832 Free PMC article.
-
Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence.Chem Rev. 2015 Oct 14;115(19):10530-74. doi: 10.1021/acs.chemrev.5b00321. Epub 2015 Aug 27. Chem Rev. 2015. PMID: 26313138 Free PMC article. Review.
References
-
- Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. J. Am. Chem. Soc. 2001;123(33):8155–8156. - PubMed
-
- Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control Release. 2001;70(1–2):1–20. - PubMed
-
- Slowing II, Trewyn BG, Lin VSY. Mesoporous silica nanoparticles for intracellular delivery of membrane-impermeable proteins. J. Am. Chem. Soc. 2007;129(28):8845–8849. - PubMed
-
- Kam NW, Dai H. Carbon nanotubes as intracellular protein transporters: generality and biological functionality. J. Am. Chem. Soc. 2005;127(16):6021–6026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

