Enabling the intestinal absorption of highly polar antiviral agents: ion-pair facilitated membrane permeation of zanamivir heptyl ester and guanidino oseltamivir
- PMID: 20536260
- PMCID: PMC3496398
- DOI: 10.1021/mp100050d
Enabling the intestinal absorption of highly polar antiviral agents: ion-pair facilitated membrane permeation of zanamivir heptyl ester and guanidino oseltamivir
Abstract
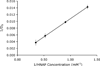
Antiviral drugs often suffer from poor intestinal permeability, preventing their delivery via the oral route. The goal of this work was to enhance the intestinal absorption of the low-permeability antiviral agents zanamivir heptyl ester (ZHE) and guanidino oseltamivir (GO) utilizing an ion-pairing approach, as a critical step toward making them oral drugs. The counterion 1-hydroxy-2-naphthoic acid (HNAP) was utilized to enhance the lipophilicity and permeability of the highly polar drugs. HNAP substantially increased the log P of the drugs by up to 3.7 log units. Binding constants (K(11(aq))) of 388 M(-1) for ZHE-HNAP and 2.91 M(-1) for GO-HNAP were obtained by applying a quasi-equilibrium transport model to double-reciprocal plots of apparent octanol-buffer distribution coefficients versus HNAP concentration. HNAP enhanced the apparent permeability (P(app)) of both compounds across Caco-2 cell monolayers in a concentration-dependent manner, as substantial P(app) (0.8-3.0 x 10(-6) cm/s) was observed in the presence of 6-24 mM HNAP, whereas no detectable transport was observed without counterion. Consistent with a quasi-equilibrium transport model, a linear relationship with slope near 1 was obtained from a log-log plot of Caco-2 P(app) versus HNAP concentration, supporting the ion-pair mechanism behind the permeability enhancement. In the rat jejunal perfusion assay, the addition of HNAP failed to increase the effective permeability (P(eff)) of GO. However, the rat jejunal permeability of ZHE was significantly enhanced by the addition of HNAP in a concentration-dependent manner, from essentially zero without HNAP to 4.0 x 10(-5) cm/s with 10 mM HNAP, matching the P(eff) of the high-permeability standard metoprolol. The success of ZHE-HNAP was explained by its >100-fold stronger K(11(aq)) versus GO-HNAP, making ZHE-HNAP less prone to dissociation and ion-exchange with competing endogenous anions and able to remain intact during membrane permeation. Overall, this work presents a novel approach to enable the oral delivery of highly polar antiviral drugs, and provides new insights into the underlying mechanisms governing the success or failure of the ion-pairing strategy to increase oral absorption.
Figures





References
-
- Kasim NA, Whitehouse M, Ramachandran C, Bermejo M, Lennernäs H, Hussain AS, Junginger HE, Stavchansky SA, Midha KK, Shah VP, Amidon GL. Molecular Properties of WHO Essential Drugs and Provisional Biopharmaceutical Classification. Mol. Pharmaceutics. 2004;1:85–96. - PubMed
-
- Takagi T, Ramachandran C, Bermejo M, Yamashita S, Yu LX, Amidon GL. A Provisional Biopharmaceutical Classification of the Top 200 Oral Drug Products in the United States, Great Britain, Spain, and Japan. Mol. Pharmaceutics. 2006;3:631–643. - PubMed
-
- Lipinski CA. Drug-Like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Meth. 2001;44:235–249. - PubMed
-
- Martinez MN, Amidon GL. A Mechanistic Approach to Understanding the Factors Affecting Drug Absorption: A Review of Fundamentals. Pharmacokin. and Pharmacodyn. 2002;42:620–643. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

