Why Drosophila to study phototransduction?
- PMID: 20536286
- PMCID: PMC2923386
- DOI: 10.3109/01677061003797302
Why Drosophila to study phototransduction?
Abstract
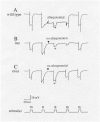
This review recounts the early history of Drosophila phototransduction genetics, covering the period between approximately 1966 to 1979. Early in this period, the author felt that there was an urgent need for a new approach in phototransduction research. Through inputs from a number of colleagues, he was led to consider isolating Drosophila mutants that are defective in the electroretinogram. Thanks to the efforts of dedicated associates and technical staff, by the end of this period, he was able to accumulate a large number of such mutants. Particularly important in this effort was the use of the mutant assay protocol based on the "prolonged depolarizing afterpotential." This collection of mutants formed the basis of the subsequent intensive investigations of the Drosophila phototransduction cascade by many investigators.
Figures




References
-
- Ahmed ST, Joyce MV, Boggess B, O'Tousa JE. The role of Drosophila ninaG oxidoreductase in visual pigment chromophore biogenesis. J. Biol. Chem. 2006;281:9205–9209. - PubMed
-
- Alderson T. Chemically induced delayed germinal mutation in Drosophila. Nature. 1965;207:164–167. - PubMed
-
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, et al. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54(5):723–733. - PubMed
-
- Brady AE, Limbird LE. G protein-coupled receptor interacting proteins: emerging roles in localization and signal transduction. Cell Signal. 2002;14:297–309. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
