Deleterious effects of freezing on osteogenic differentiation of human adipose-derived stromal cells in vitro and in vivo
- PMID: 20536327
- PMCID: PMC3128779
- DOI: 10.1089/scd.2010.0082
Deleterious effects of freezing on osteogenic differentiation of human adipose-derived stromal cells in vitro and in vivo
Abstract
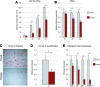
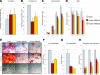
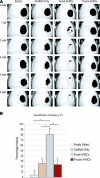
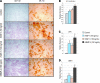
Human adipose-derived stromal cells (hASCs) represent a multipotent stromal cell type with a proven capacity to undergo osteogenic differentiation. Many hurdles exist, however, between current knowledge of hASC osteogenesis and their potential future use in skeletal tissue regeneration. The impact of frozen storage on hASC osteogenic differentiation, for example, has not been studied in detail. To examine the effects of frozen storage, hASCs were harvested from lipoaspirate and either maintained in standard culture conditions or frozen for 2 weeks under standard conditions (90% fetal bovine serum, 10% dimethyl sulfoxide). Next, in vitro parameters of cell morphology (surface electron microscopy [EM]), cell viability and growth (trypan blue; bromodeoxyuridine incorporation), osteogenic differentiation (alkaline phosphatase, alizarin red, and quantitative real-time (RT)-polymerase chain reaction), and adipogenic differentiation (Oil red O staining and quantitative RT-polymerase chain reaction) were performed. Finally, in vivo bone formation was assessed using a critical-sized cranial defect in athymic mice, utilizing a hydroxyapatite (HA)-poly(lactic-co-glycolic acid) scaffold for ASC delivery. Healing was assessed by serial microcomputed tomography scans and histology. Freshly derived ASCs differed significantly from freeze-thaw ASCs in all markers examined. Surface EM showed distinct differences in cellular morphology. Proliferation, and osteogenic and adipogenic differentiation were all significantly hampered by the freeze-thaw process in vitro (*P < 0.01). In vivo, near complete healing was observed among calvarial defects engrafted with fresh hASCs. This was in comparison to groups engrafted with freeze-thaw hASCs that showed little healing (*P < 0.01). Finally, recombinant insulin-like growth factor 1 or recombinant bone morphogenetic protein 4 was observed to increase or rescue in vitro osteogenic differentiation among frozen hASCs (*P < 0.01). The freezing of ASCs for storage significantly impacts their biology, both in vitro and in vivo. The ability of ASCs to successfully undergo osteogenic differentiation after freeze-thaw is substantively muted, both in vitro and in vivo. The use of recombinant proteins, however, may be used to mitigate the deleterious effects of the freeze-thaw process.
Figures



Similar articles
-
Human adipose-derived stromal cells stimulate autogenous skeletal repair via paracrine Hedgehog signaling with calvarial osteoblasts.Stem Cells Dev. 2011 Feb;20(2):243-57. doi: 10.1089/scd.2010.0250. Epub 2010 Oct 12. Stem Cells Dev. 2011. PMID: 20698749 Free PMC article.
-
Human adipose derived stromal cells heal critical size mouse calvarial defects.PLoS One. 2010 Jun 17;5(6):e11177. doi: 10.1371/journal.pone.0011177. PLoS One. 2010. PMID: 20567510 Free PMC article.
-
[Osteogenic capability of primary human adipose-derived stromal cells in vivo].Beijing Da Xue Xue Bao Yi Xue Ban. 2012 Feb 18;44(1):55-8. Beijing Da Xue Xue Bao Yi Xue Ban. 2012. PMID: 22353901 Chinese.
-
Current status of human adipose-derived stem cells: differentiation into hepatocyte-like cells.ScientificWorldJournal. 2011;11:1568-81. doi: 10.1100/tsw.2011.146. Epub 2011 Aug 16. ScientificWorldJournal. 2011. PMID: 22224071 Free PMC article. Review.
-
Current applications of adipose-derived mesenchymal stem cells in bone repair and regeneration: A review of cell experiments, animal models, and clinical trials.Front Bioeng Biotechnol. 2022 Sep 7;10:942128. doi: 10.3389/fbioe.2022.942128. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36159705 Free PMC article. Review.
Cited by
-
Single and repeated intra-articular injections in the tarsocrural joint with allogeneic and autologous equine bone marrow-derived mesenchymal stem cells are safe, but did not reduce acute inflammation in an experimental interleukin-1β model of synovitis.Equine Vet J. 2020 Jul;52(4):601-612. doi: 10.1111/evj.13222. Epub 2020 Feb 14. Equine Vet J. 2020. PMID: 31821594 Free PMC article.
-
Current methods of adipogenic differentiation of mesenchymal stem cells.Stem Cells Dev. 2011 Oct;20(10):1793-804. doi: 10.1089/scd.2011.0040. Epub 2011 Jun 20. Stem Cells Dev. 2011. PMID: 21526925 Free PMC article. Review.
-
Enhanced osteogenesis of adipose derived stem cells with Noggin suppression and delivery of BMP-2.PLoS One. 2013 Aug 15;8(8):e72474. doi: 10.1371/journal.pone.0072474. eCollection 2013. PLoS One. 2013. PMID: 23977305 Free PMC article.
-
A comparison of bone regeneration with human mesenchymal stem cells and muscle-derived stem cells and the critical role of BMP.Biomaterials. 2014 Aug;35(25):6859-70. doi: 10.1016/j.biomaterials.2014.04.113. Epub 2014 May 21. Biomaterials. 2014. PMID: 24856105 Free PMC article.
-
Concise review: adipose-derived stromal cells for skeletal regenerative medicine.Stem Cells. 2011 Apr;29(4):576-82. doi: 10.1002/stem.612. Stem Cells. 2011. PMID: 21305671 Free PMC article. Review.
References
-
- Kwan MD. Slater BJ. Wan DC. Longaker MT. Cell-based therapies for skeletal regenerative medicine. Hum Mol Genet. 2008;17:R93–R98. - PubMed
-
- Zuk PA. Zhu M. Mizuno H. Huang J. Futrell JW. Katz AJ. Benhaim P. Lorenz HP. Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. - PubMed
-
- Wan DC. Pomerantz JH. Brunet LJ. Kim JB. Chou YF. Wu BM. Harland R. Blau HM. Longaker MT. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem. 2007;282:26450–26459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

