Deleterious effects of freezing on osteogenic differentiation of human adipose-derived stromal cells in vitro and in vivo
- PMID: 20536327
- PMCID: PMC3128779
- DOI: 10.1089/scd.2010.0082
Deleterious effects of freezing on osteogenic differentiation of human adipose-derived stromal cells in vitro and in vivo
Abstract
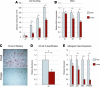
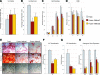
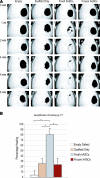
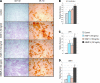
Human adipose-derived stromal cells (hASCs) represent a multipotent stromal cell type with a proven capacity to undergo osteogenic differentiation. Many hurdles exist, however, between current knowledge of hASC osteogenesis and their potential future use in skeletal tissue regeneration. The impact of frozen storage on hASC osteogenic differentiation, for example, has not been studied in detail. To examine the effects of frozen storage, hASCs were harvested from lipoaspirate and either maintained in standard culture conditions or frozen for 2 weeks under standard conditions (90% fetal bovine serum, 10% dimethyl sulfoxide). Next, in vitro parameters of cell morphology (surface electron microscopy [EM]), cell viability and growth (trypan blue; bromodeoxyuridine incorporation), osteogenic differentiation (alkaline phosphatase, alizarin red, and quantitative real-time (RT)-polymerase chain reaction), and adipogenic differentiation (Oil red O staining and quantitative RT-polymerase chain reaction) were performed. Finally, in vivo bone formation was assessed using a critical-sized cranial defect in athymic mice, utilizing a hydroxyapatite (HA)-poly(lactic-co-glycolic acid) scaffold for ASC delivery. Healing was assessed by serial microcomputed tomography scans and histology. Freshly derived ASCs differed significantly from freeze-thaw ASCs in all markers examined. Surface EM showed distinct differences in cellular morphology. Proliferation, and osteogenic and adipogenic differentiation were all significantly hampered by the freeze-thaw process in vitro (*P < 0.01). In vivo, near complete healing was observed among calvarial defects engrafted with fresh hASCs. This was in comparison to groups engrafted with freeze-thaw hASCs that showed little healing (*P < 0.01). Finally, recombinant insulin-like growth factor 1 or recombinant bone morphogenetic protein 4 was observed to increase or rescue in vitro osteogenic differentiation among frozen hASCs (*P < 0.01). The freezing of ASCs for storage significantly impacts their biology, both in vitro and in vivo. The ability of ASCs to successfully undergo osteogenic differentiation after freeze-thaw is substantively muted, both in vitro and in vivo. The use of recombinant proteins, however, may be used to mitigate the deleterious effects of the freeze-thaw process.
Figures



References
-
- Kwan MD. Slater BJ. Wan DC. Longaker MT. Cell-based therapies for skeletal regenerative medicine. Hum Mol Genet. 2008;17:R93–R98. - PubMed
-
- Zuk PA. Zhu M. Mizuno H. Huang J. Futrell JW. Katz AJ. Benhaim P. Lorenz HP. Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. - PubMed
-
- Wan DC. Pomerantz JH. Brunet LJ. Kim JB. Chou YF. Wu BM. Harland R. Blau HM. Longaker MT. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem. 2007;282:26450–26459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

