Macrocyclic inhibitors of hsp90
- PMID: 20536417
- PMCID: PMC3105290
- DOI: 10.2174/156802610792232088
Macrocyclic inhibitors of hsp90
Abstract
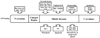
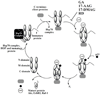
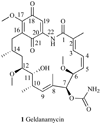
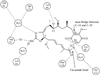
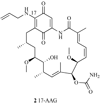
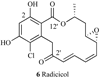
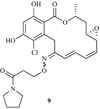
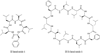
Heat shock proteins (HSP) are a family of highly conserved proteins, whose expression increases in response to stresses that may threaten cell survival. Over the past decade, heat shock protein 90 (Hsp90) has emerged as a potential therapeutic target for cancer as it plays a vital role in normal cell maturation and acts as a molecular chaperone for proper folding, assembly, and stabilization of many oncogenic proteins. To date, a majority of Hsp90 inhibitors that have been discovered are macrocycles. The relatively rigid conformation provided by the macrocyclic scaffold allows for a selective interaction with a biological target such as Hsp90. This review highlights the discovery and development of nine macrocycles that inhibit the function of Hsp90, detailing their potency and the client proteins affected by Hsp90 inhibition.
Figures


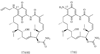
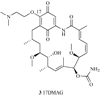
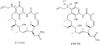
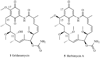






References
-
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29. - PubMed
-
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol:from nascent chain to folded protein. Science. 2002;295:1852–1858. - PubMed
-
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993;27:437–496. - PubMed
-
- Welch WJ, Feramisco JR. Purification of the major mammalian heat shock proteins. J. Biol. Chem. 1982;257(24):14949–14959. - PubMed
-
- Crevel G, Bates H, Huikeshoven H, Cotterill S. The Drosophila Dpit47 protein is a nuclear Hsp90 co-chaperone that interacts with DNA polymerase alpha. J. Cell Sci. 2001;114(Pt 11):2015–2025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

