The role of mTOR in memory CD8 T-cell differentiation
- PMID: 20536567
- PMCID: PMC3760155
- DOI: 10.1111/j.0105-2896.2010.00898.x
The role of mTOR in memory CD8 T-cell differentiation
Abstract
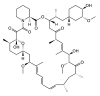
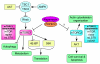
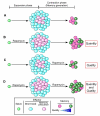
The mammalian target of rapamycin (mTOR) is an intracellular kinase that regulates cell growth and metabolism. Its specific inhibitor rapamycin is currently used in transplant recipients as an immunosuppressive drug to prevent allograft rejection. Studies have shown complex and diverse mechanisms for the immunosuppressive effects of rapamycin. The drug has been reported to inhibit T-cell proliferation, induce anergy, modulate T-cell trafficking, promote regulatory T cells, and also prevent maturation of dendritic cells as well as production of type I interferon. However, several other studies have paradoxically demonstrated immunostimulatory effects of rapamycin by improving antigen presentation and regulating cytokine production from macrophages and myeloid dendritic cells. Recently, it has been shown that rapamycin also exhibits immunostimulatory effects on memory CD8(+) T-cell differentiation. The drug improved both quantity and quality of memory CD8(+) T cells induced by viral infection and vaccination, showing that mTOR is a major regulator of memory CD8(+) T-cell differentiation. These discoveries have implications for the development of novel vaccine regimens. Here, we review the role of mTOR in memory CD8(+) T-cell differentiation and compare the effect of rapamycin among CD8(+) T cells, CD4(+) T cells, and dendritic cells. Also, we discuss potential application of these findings in a clinical setting.
Figures

References
-
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
-
- Masopust D. Developing an HIV cytotoxic T-lymphocyte vaccine: issues of CD8 T-cell quantity, quality and location. J Intern Med. 2009;265:125–137. - PubMed
-
- Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

