Proteomic analysis of shear stress-mediated protection from TNF-alpha in endothelial cells
- PMID: 20536739
- PMCID: PMC3712086
- DOI: 10.1111/j.1549-8719.2010.00031.x
Proteomic analysis of shear stress-mediated protection from TNF-alpha in endothelial cells
Abstract
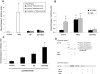
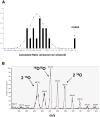
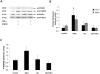
Previous studies have shown that physiological levels of shear stress can protect endothelial cells (ECs) from apoptotic stimuli. Here, we differentiate between acute and chronic protection and demonstrate the use of proteomic technologies to uncover mechanisms associated with chronic protection of ECs. We hypothesized that changes in abundance of proteins associated with the TNF-alpha signaling cascade orchestrate shear stress-mediated protection from TNF-alpha when cells are preconditioned with shear prior to the exposure of apoptotic stimuli. Detection of cleaved caspase 3 through Western blot analysis confirmed chronic shear stress-mediated protection from TNF-alpha. In the presence of the nitric oxide synthase inhibitor, LNMA (N(omega)-monomethyl-l-arginine), chronic protection remained. Treatment with a de novo protein synthesis inhibitor, cycloheximide, eliminated this protective effect. Isotopic-labeling experiments, coupled with LC-MS/MS (liquid chromatography-tandem mass spectrometry) of isolated components of the TNF-alpha pathway revealed that CARD9, a known activator of the NF-kappaB pathway, was increased (60%) in sheared cells versus nonsheared cells. This result was confirmed through Western blot analysis. Our data suggest that de novo formation of proteins is required for protection from TNF-alpha in ECs chronically exposed to shear stress, and that CARD9 is a candidate protein in this response.
Figures
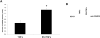
Similar articles
-
NF-kappaB stimulates inducible nitric oxide synthase to protect mouse hepatocytes from TNF-alpha- and Fas-mediated apoptosis.Gastroenterology. 2001 Apr;120(5):1251-62. doi: 10.1053/gast.2001.23239. Gastroenterology. 2001. PMID: 11266388
-
A20 protects endothelial cells from TNF-, Fas-, and NK-mediated cell death by inhibiting caspase 8 activation.Blood. 2004 Oct 15;104(8):2376-84. doi: 10.1182/blood-2003-02-0635. Epub 2004 Jul 13. Blood. 2004. PMID: 15251990
-
Inhibitory action of nitric oxide on circulating tumor necrosis factor-induced NF-kappaB activity and COX-2 transcription in the endothelium of the brain capillaries.J Neuropathol Exp Neurol. 2001 Sep;60(9):893-905. doi: 10.1093/jnen/60.9.893. J Neuropathol Exp Neurol. 2001. PMID: 11556546
-
Shear stress attenuates tumor necrosis factor-alpha-induced monocyte chemotactic protein-1 expressions in endothelial cells.Chin J Physiol. 2002 Dec 31;45(4):169-76. Chin J Physiol. 2002. PMID: 12817708
-
Role of NF-kappaB and PI 3-kinase/Akt in TNF-alpha-induced cytotoxicity in microvascular endothelial cells.Am J Physiol Renal Physiol. 2008 Oct;295(4):F932-41. doi: 10.1152/ajprenal.00066.2008. Epub 2008 Jul 16. Am J Physiol Renal Physiol. 2008. PMID: 18632801
Cited by
-
CARD9 Signaling, Inflammation, and Diseases.Front Immunol. 2022 Mar 30;13:880879. doi: 10.3389/fimmu.2022.880879. eCollection 2022. Front Immunol. 2022. PMID: 35432375 Free PMC article. Review.
-
Shear stress-initiated signaling and its regulation of endothelial function.Arterioscler Thromb Vasc Biol. 2014 Oct;34(10):2191-8. doi: 10.1161/ATVBAHA.114.303422. Epub 2014 May 29. Arterioscler Thromb Vasc Biol. 2014. PMID: 24876354 Free PMC article. Review.
-
Vascular endothelial growth factor-A signaling in bone marrow-derived endothelial progenitor cells exposed to hypoxic stress.Physiol Genomics. 2013 Nov 1;45(21):1021-34. doi: 10.1152/physiolgenomics.00070.2013. Epub 2013 Sep 10. Physiol Genomics. 2013. PMID: 24022223 Free PMC article.
-
Deletion of hematopoietic Dectin-2 or CARD9 does not protect against atherosclerotic plaque formation in hyperlipidemic mice.Sci Rep. 2019 Mar 13;9(1):4337. doi: 10.1038/s41598-019-40663-x. Sci Rep. 2019. PMID: 30867470 Free PMC article.
-
Omics-based approaches to understand mechanosensitive endothelial biology and atherosclerosis.Wiley Interdiscip Rev Syst Biol Med. 2016 Sep;8(5):378-401. doi: 10.1002/wsbm.1344. Epub 2016 Jun 24. Wiley Interdiscip Rev Syst Biol Med. 2016. PMID: 27341633 Free PMC article. Review.
References
-
- Abdel-Mageed AB, Agrawal KC. Activation of nuclear factor kappaB: potential role in metallothionein-mediated mitogenic response. Cancer Res. 1998;58:2335–2338. - PubMed
-
- Abumiya T, Sasaguri T, Taba Y, Miwa Y, Miyagi M. Shear stress induces expression of vascular endothelial growth factor receptor Flk-1/KDR through the CT-rich Sp1 binding site. Arterioscler Thromb Vasc Biol. 2002;22:907–913. - PubMed
-
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. - PubMed
-
- Bertin J, Guo Y, Wang L, Srinivasula SM, Jacobson MD, Poyet JL, Merriam S, Du MQ, Dyer MJ, Robison KE, DiStefano PS, Alnemri ES. CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-kappa B. J Biol Chem. 2000;275:41082–41086. - PubMed
-
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

