The role of T cell PPAR gamma in mice with experimental inflammatory bowel disease
- PMID: 20537136
- PMCID: PMC2891618
- DOI: 10.1186/1471-230X-10-60
The role of T cell PPAR gamma in mice with experimental inflammatory bowel disease
Abstract
Background: Peroxisome proliferator-activated receptor gamma (PPAR gamma) is a nuclear receptor whose activation has been shown to modulate macrophage and T cell-mediated inflammation. The objective of this study was to investigate the mechanisms by which the deletion of PPAR gamma in T cells modulates immune cell distribution and colonic gene expression and the severity of experimental IBD.
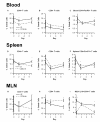
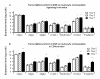
Methods: PPAR gamma flfl; CD4 Cre+ (CD4cre) or Cre- (WT) mice were challenged with 2.5% dextran sodium sulfate in their drinking water for 0, 2, or 7 days. Mice were scored on disease severity both clinically and histopathologically. Flow cytometry was used to assess lymphocyte and macrophage populations in the blood, spleen, and mesenteric lymph nodes (MLN). Global gene expression in colonic mucosa was profiled using Affymetrix microarrays.
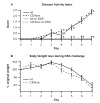
Results: The deficiency of PPAR gamma in T cells accelerated the onset of disease and body weight loss. Examination of colon histopathology revealed significantly greater epithelial erosion, leukocyte infiltration, and mucosal thickening in the CD4cre mice on day 7. CD4cre mice had more CD8+ T cells than WT mice and fewer CD4+ FoxP3+ regulatory T cells (Treg) and IL10+ CD4+ T cells in blood and MLN, respectively. Transcriptomic profiling revealed around 3000 genes being transcriptionally altered as a result of DSS challenge in CD4cre mice. These included up-regulated mRNA expression of adhesion molecules, proinflammatory cytokines interleukin-6 (IL-6) and IL-1beta, and suppressor of cytokine signaling 3 (SOCS-3) on day 7. Gene set enrichment analysis (GSEA) showed that the ribosome and Krebs cycle pathways were downregulated while the apoptosis pathway was upregulated in colons of mice lacking PPAR gamma in T cells.
Conclusions: The expression of PPAR gamma in T cells is involved in preventing gut inflammation by regulating colonic expression of adhesion molecules and inflammatory mediators at later stages of disease while favoring the recruitment of Treg to the mucosal inductive sites.
Figures


References
-
- Bassaganya-Riera J, Reynolds K, Martino-Catt S, Cui Y, Hennighausen L, Gonzalez F, Rohrer J, Benninghoff AU, Hontecillas R. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127(3):777–791. doi: 10.1053/j.gastro.2004.06.049. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

