Strategy escalation: an emerging paradigm for safe clinical development of T cell gene therapies
- PMID: 20537174
- PMCID: PMC2904270
- DOI: 10.1186/1479-5876-8-55
Strategy escalation: an emerging paradigm for safe clinical development of T cell gene therapies
Abstract
Gene therapy techniques are being applied to modify T cells with chimeric antigen receptors (CARs) for therapeutic ends. The versatility of this platform has spawned multiple options for their application with new permutations in strategies continually being invented, a testimony to the creative energies of many investigators. The field is rapidly expanding with immense potential for impact against diverse cancers. But this rapid expansion, like the Big Bang, comes with a somewhat chaotic evolution of its therapeutic universe that can also be dangerous, as seen by recently publicized deaths. Time-honored methods for new drug testing embodied in Dose Escalation that were suitable for traditional inert agents are now inadequate for these novel "living drugs". In the following, I propose an approach to escalating risk for patient exposures with these new immuno-gene therapy agents, termed Strategy Escalation, that accounts for the molecular and biological features of the modified cells and the methods of their administration. This proposal is offered not as a prescriptive but as a discussion framework that investigators may wish to consider in configuring their intended clinical applications.
Figures
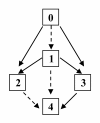
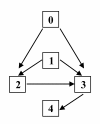
Similar articles
-
The promise and potential pitfalls of chimeric antigen receptors.Curr Opin Immunol. 2009 Apr;21(2):215-23. doi: 10.1016/j.coi.2009.02.009. Epub 2009 Mar 25. Curr Opin Immunol. 2009. PMID: 19327974 Free PMC article. Review.
-
Gene-modified hematopoietic stem cells for cancer immunotherapy.Hum Vaccin Immunother. 2014;10(4):982-5. doi: 10.4161/hv.27637. Epub 2014 Jan 9. Hum Vaccin Immunother. 2014. PMID: 24398603 Free PMC article.
-
Strategies for enhancing adoptive T-cell immunotherapy against solid tumors using engineered cytokine signaling and other modalities.Expert Opin Biol Ther. 2018 Jun;18(6):653-664. doi: 10.1080/14712598.2018.1473368. Epub 2018 May 14. Expert Opin Biol Ther. 2018. PMID: 29727246 Free PMC article. Review.
-
Immunotherapy of acute leukemia by chimeric antigen receptor-modified lymphocytes using an improved Sleeping Beauty transposon platform.Oncotarget. 2016 Aug 9;7(32):51581-51597. doi: 10.18632/oncotarget.9955. Oncotarget. 2016. PMID: 27323395 Free PMC article.
-
Minicircle-Based Engineering of Chimeric Antigen Receptor (CAR) T Cells.Recent Results Cancer Res. 2016;209:37-50. doi: 10.1007/978-3-319-42934-2_3. Recent Results Cancer Res. 2016. PMID: 28101686 Review.
Cited by
-
Is it safer CARs that we need, or safer rules of the road?Mol Ther. 2010 Oct;18(10):1742-3. doi: 10.1038/mt.2010.162. Mol Ther. 2010. PMID: 20885432 Free PMC article. No abstract available.
-
Considerations for the clinical application of chimeric antigen receptor T cells: observations from a recombinant DNA Advisory Committee Symposium held June 15, 2010.Cancer Res. 2011 May 1;71(9):3175-81. doi: 10.1158/0008-5472.CAN-10-4035. Cancer Res. 2011. PMID: 21531763 Free PMC article.
-
The risk-escalation model: a principled design strategy for early-phase trials.Kennedy Inst Ethics J. 2014 Jun;24(2):121-39. doi: 10.1353/ken.2014.0017. Kennedy Inst Ethics J. 2014. PMID: 25109092 Free PMC article.
-
The challenges of solid tumor for designer CAR-T therapies: a 25-year perspective.Cancer Gene Ther. 2017 Mar;24(3):89-99. doi: 10.1038/cgt.2016.82. Cancer Gene Ther. 2017. PMID: 28392558 No abstract available.
-
Chimeric NKG2D CAR-expressing T cell-mediated attack of human ovarian cancer is enhanced by histone deacetylase inhibition.Hum Gene Ther. 2013 Mar;24(3):295-305. doi: 10.1089/hum.2012.143. Epub 2013 Mar 1. Hum Gene Ther. 2013. PMID: 23297870 Free PMC article.
References
-
- Ma QZ, Gonzalo-Daganzo R, Junghans RP. In: Cancer Chemotherapy & Biological Response Modifiers - Annual 20. Giaccone R, Schlinsky R, Sondel P, editor. Oxford: Elsevier Science; 2002. Genetically engineered T cells as adoptive immunotherapy of cancer; pp. 319–45. - PubMed
-
- Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–2. doi: 10.1200/JCO.2006.05.9964. - DOI - PubMed
-
- Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, White DE, Steinberg SM. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–80. - PubMed
-
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–15. doi: 10.1158/1078-0432.CCR-06-1183. - DOI - PMC - PubMed
-
- Warren RS, Fisher GA, Bergaland EK, Pennathur-Das R, Nemunaitis J, Venook AP, Hege KM. Studies of regional and systemic gene therapy with autologous CC49-zeta modified T cells in colorectal cancer metastatic to liver. (Abstract, 7th International Conference on Gene Therapy of Cancer) Cancer Gene Ther. 1998;5:S1–S2. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

