Liver fatty acid-binding protein and obesity
- PMID: 20537520
- PMCID: PMC2939181
- DOI: 10.1016/j.jnutbio.2010.01.005
Liver fatty acid-binding protein and obesity
Abstract
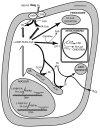
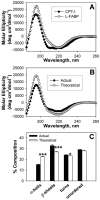
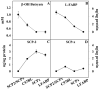
While low levels of unesterified long chain fatty acids (LCFAs) are normal metabolic intermediates of dietary and endogenous fat, LCFAs are also potent regulators of key receptors/enzymes and at high levels become toxic detergents within the cell. Elevated levels of LCFAs are associated with diabetes, obesity and metabolic syndrome. Consequently, mammals evolved fatty acid-binding proteins (FABPs) that bind/sequester these potentially toxic free fatty acids in the cytosol and present them for rapid removal in oxidative (mitochondria, peroxisomes) or storage (endoplasmic reticulum, lipid droplets) organelles. Mammals have a large (15-member) family of FABPs with multiple members occurring within a single cell type. The first described FABP, liver-FABP (L-FABP or FABP1), is expressed in very high levels (2-5% of cytosolic protein) in liver as well as in intestine and kidney. Since L-FABP facilitates uptake and metabolism of LCFAs in vitro and in cultured cells, it was expected that abnormal function or loss of L-FABP would reduce hepatic LCFA uptake/oxidation and thereby increase LCFAs available for oxidation in muscle and/or storage in adipose. This prediction was confirmed in vitro with isolated liver slices and cultured primary hepatocytes from L-FABP gene-ablated mice. Despite unaltered food consumption when fed a control diet ad libitum, the L-FABP null mice exhibited age- and sex-dependent weight gain and increased fat tissue mass. The obese phenotype was exacerbated in L-FABP null mice pair fed a high-fat diet. Taken together with other findings, these data suggest that L-FABP could have an important role in preventing age- or diet-induced obesity.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Ockner RK, Manning JA, Poppenhausen RB, Ho WK. A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science. 1972;177:56–58. - PubMed
-
- Glatz JF, Veerkamp JH. Intracellular fatty acid-binding proteins. [Review] Int J Biochem. 1985;17:13–22. - PubMed
-
- Glatz JFC, Van der Vusse GJ, Veerkamp JH. Fatty acid-binding proteins and their physiological significance. New Physiol Sci. 1988;3:41–43.
-
- Paulussen RJA, Veerkamp JH. Intracellular fatty acid-binding proteins characteristics and function. In: Hilderson HJ, editor. Subcellular Biochemistry. New York: Plenum Press; 1990. pp. 175–226. - PubMed
-
- Veerkamp JH, van Moerkerk HT. Fatty acid-binding protein and its relation to fatty acid oxidation. Mol Cell Biochem. 1993;123:101–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

