Dynamic imaging reveals that brain-derived neurotrophic factor can independently regulate motility and direction of neuroblasts within the rostral migratory stream
- PMID: 20538046
- PMCID: PMC2935908
- DOI: 10.1016/j.neuroscience.2010.05.075
Dynamic imaging reveals that brain-derived neurotrophic factor can independently regulate motility and direction of neuroblasts within the rostral migratory stream
Abstract
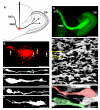
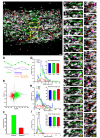
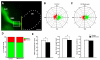
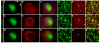
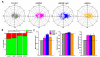
Neuronal precursors generated in the subventricular zone (SVZ) migrate through the rostral migratory stream (RMS) to the olfactory bulb (OB). Although, the mechanisms regulating this migration remain largely unknown. Studies have shown that molecular factors, such as brain-derived neurotrophic factor (BDNF) emanating from the OB, may function as chemoattractants drawing neuroblasts toward their target. To better understand the role of BDNF in RMS migration, we used an acute slice preparation from early postnatal mice to track the tangential migration of GAD65-GFP labeled RMS neuroblasts with confocal time-lapse imaging. By quantifying the cell dynamics using specific directional and motility criteria, our results showed that removal of the OB did not alter the overall directional trajectory of neuroblasts, but did reduce their motility. This suggested that additional guidance factors present locally within the RMS region also contribute to this migration. Here we report that BDNF and its high affinity receptor, tyrosine kinase receptor type 2 (TrkB), are indeed heterogeneously expressed within the RMS at postnatal day 7. By altering BDNF levels within the entire pathway, we showed that reduced BDNF signaling changes both neuroblast motility and direction, while increased BDNF levels changes only motility. Together these data reveal that during this early postnatal period BDNF plays a complex role in regulating both the motility and direction of RMS flow, and that BDNF comes from sources within the RMS itself, as well as from the olfactory bulb.
Published by Elsevier Ltd.
Figures

References
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. - PubMed
-
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. - PubMed
-
- Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, Proenca CC, Kraemer R, Cleland TA, Hempstead BL, Chao MV, Lee FS. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28:2383–2393. - PMC - PubMed
-
- Bovetti S, Bovolin P, Perroteau I, Puche AC. Subventricular zone-derived neuroblast migration to the olfactory bulb is modulated by matrix remodelling. Eur J Neurosci. 2007;25:2021–2033. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

