Getting the mOST from OST: Role of organic solute transporter, OSTalpha-OSTbeta, in bile acid and steroid metabolism
- PMID: 20538072
- PMCID: PMC2911127
- DOI: 10.1016/j.bbalip.2010.06.002
Getting the mOST from OST: Role of organic solute transporter, OSTalpha-OSTbeta, in bile acid and steroid metabolism
Abstract
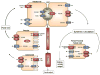
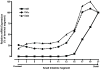
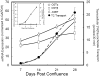
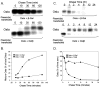
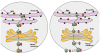
The organic solute transporter (OST)(alpha)-OST(beta) is an unusual heteromeric carrier expressed in a variety of tissues including the small intestine, colon, liver, biliary tract, kidney, and adrenal gland. In polarized epithelial cells, OSTalpha-OSTbeta protein is localized on the basolateral membrane and functions in the export or uptake of bile acids and steroids. This article reviews recent results including studies of knockout mouse models that provide new insights to the role of OSTalpha-OSTbeta in the compartmentalization and metabolism of these important lipids.
2010 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Pleiotropic functions of the organic solute transporter Ostα-Ostβ.Dig Dis. 2011;29(1):13-7. doi: 10.1159/000324123. Epub 2011 Jun 17. Dig Dis. 2011. PMID: 21691099 Free PMC article. Review.
-
OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia.Hepatology. 2005 Dec;42(6):1270-9. doi: 10.1002/hep.20961. Hepatology. 2005. PMID: 16317684
-
The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter.J Biol Chem. 2005 Feb 25;280(8):6960-8. doi: 10.1074/jbc.M412752200. Epub 2004 Nov 24. J Biol Chem. 2005. PMID: 15563450 Free PMC article.
-
Ostalpha-Ostbeta is required for bile acid and conjugated steroid disposition in the intestine, kidney, and liver.Am J Physiol Gastrointest Liver Physiol. 2008 Jul;295(1):G179-G186. doi: 10.1152/ajpgi.90319.2008. Epub 2008 May 22. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18497332 Free PMC article.
-
Organic solute transporter, OSTalpha-OSTbeta: its role in bile acid transport and cholestasis.Semin Liver Dis. 2010 May;30(2):178-85. doi: 10.1055/s-0030-1253226. Epub 2010 Apr 26. Semin Liver Dis. 2010. PMID: 20422499 Free PMC article. Review.
Cited by
-
Heteromeric Solute Carriers: Function, Structure, Pathology and Pharmacology.Adv Exp Med Biol. 2021;21:13-127. doi: 10.1007/5584_2020_584. Adv Exp Med Biol. 2021. PMID: 33052588 Review.
-
Pleiotropic functions of the organic solute transporter Ostα-Ostβ.Dig Dis. 2011;29(1):13-7. doi: 10.1159/000324123. Epub 2011 Jun 17. Dig Dis. 2011. PMID: 21691099 Free PMC article. Review.
-
Pectin Penta-Oligogalacturonide Suppresses Intestinal Bile Acids Absorption and Downregulates the FXR-FGF15 Axis in High-Cholesterol Fed Mice.Lipids. 2017 Jun;52(6):489-498. doi: 10.1007/s11745-017-4258-x. Epub 2017 May 4. Lipids. 2017. PMID: 28474246
-
Host metabolism dysregulation and cell tropism identification in human airway and alveolar organoids upon SARS-CoV-2 infection.Protein Cell. 2021 Sep;12(9):717-733. doi: 10.1007/s13238-020-00811-w. Epub 2020 Dec 12. Protein Cell. 2021. PMID: 33314005 Free PMC article.
-
Pleiotropic roles of bile acids in metabolism.Cell Metab. 2013 May 7;17(5):657-69. doi: 10.1016/j.cmet.2013.03.013. Epub 2013 Apr 18. Cell Metab. 2013. PMID: 23602448 Free PMC article. Review.
References
-
- Lin MC, Weinberg SL, Kramer W, Burckhardt G, Wilson FA. Identification and comparison of bile acid-binding polypeptides in ileal basolateral membrane. J Membr Biol. 1988;106:1–11. - PubMed
-
- Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3) J Biol Chem. 2000;275:2905–2910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

