Synthesis, structure-activity relationship and antiviral activity of 3'-N,N-dimethylamino-2',3'-dideoxythymidine and its prodrugs
- PMID: 20538384
- PMCID: PMC7127469
- DOI: 10.1016/j.ejmech.2010.05.028
Synthesis, structure-activity relationship and antiviral activity of 3'-N,N-dimethylamino-2',3'-dideoxythymidine and its prodrugs
Abstract
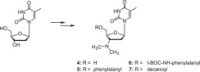
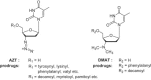
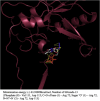
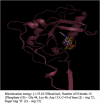
A probable NRTI molecule, viz. 3'-N,N-dimethylamino-2',3'-dideoxythymidine (4) and its 5'-O-carboxyl ester prodrugs - 5'-(N-alpha-BOC-L-phenylalanyl)-3'-N,N-dimethylamino-2',3'-dideoxythymidine (5), 5'-L-phenylalanyl-3'-N,N-dimethylamino-2',3'-dideoxythymidine (6) and 5'-decanoyl-3'-N,N-dimethylamino-2',3'-dideoxythymidine (7) have been synthesized and screened against HIV, HSV-1 and 2, parainfluenza-3, vesicular stomatitis and several other viruses. The compound 6 showed good antiviral activity with EC(50) value 0.03 microM (SI=8) against VSV in Hela and HEL cell lines. However, the lead compound 4 and its derivatives 5, 6 and 7 showed no remarkable activity against HIV-1 and other viruses. Molecular docking studies with HIV-1 RT using DS 2.5 and pymol softwares have shown marked differences in the interaction patterns between the lead compound 4 and AZT.
2010 Elsevier Masson SAS. All rights reserved.
Figures


References
-
- Ali H., Ahmed N., Tessier G., Lier J.E.V. Bioorg. Med. Chem. Lett. 2006;15:317–319. - PubMed
-
- Genini D., Adachi S., Chao Q., Rose D.W., Carrera C.J., Kottam H.B., Carson D., Leoni L.M. Blood. 2000;96:3537–3543. - PubMed
-
- Gudumundsson K.S., Freeman G.A., Drach J.C., Townsend L.B. J. Med. Chem. 2000;43:2473–2478. - PubMed
-
- Lee Y.S., Kim B.H. Bioorg. Med. Chem. Lett. 2002;20:1395–1397. - PubMed
-
- Galmarini C.M., Mackey J.R., Dumontet C. Nature. 2001;15:875–890. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

