Novel recombinant engineered gp41 N-terminal heptad repeat trimers and their potential as anti-HIV-1 therapeutics or microbicides
- PMID: 20538590
- PMCID: PMC2919114
- DOI: 10.1074/jbc.M110.101170
Novel recombinant engineered gp41 N-terminal heptad repeat trimers and their potential as anti-HIV-1 therapeutics or microbicides
Abstract
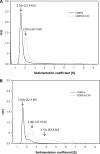
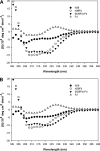
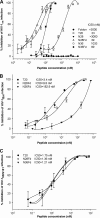
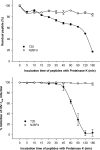
Peptides derived from N-terminal heptad repeat (NHR) of the HIV-1 gp41 are generally poor inhibitors of HIV-1 entry, because they tend to aggregate and do not form a trimeric coiled-coil. In this study, we have fused portions of gp41 NHR, e.g. N36 or N28, to the T4 fibritin trimerization domain, Foldon (Fd), thus constructing novel NHR trimers, designated N36Fd or N28Fd, which could be expressed in Escherichia coli cells. The purified N36Fd and N28Fd exhibited SDS-resistant trimeric coiled-coil conformation with improved alpha-helicity compared with the corresponding N-peptides. They could interact with a C-peptide (e.g. C34) to form stable six-helix bundle and possessed potent anti-HIV-1 activity against a broad spectrum of HIV-1 strains. N28Fd was effective against T20-resistant HIV-1 variants and more resistant to proteinase K compared with T20 (enfuvirtide), a C-peptide-based HIV fusion inhibitor. Therefore, N28Fd trimer has great potentials for further development as an affordable therapeutic or microbicide for treatment and prevention of HIV-1 infection.
Figures




Similar articles
-
An engineered HIV-1 gp41 trimeric coiled coil with increased stability and anti-HIV-1 activity: implication for developing anti-HIV microbicides.J Antimicrob Chemother. 2013 Nov;68(11):2533-44. doi: 10.1093/jac/dkt230. Epub 2013 Jun 21. J Antimicrob Chemother. 2013. PMID: 23794600
-
HIV-1 variants with a single-point mutation in the gp41 pocket region exhibiting different susceptibility to HIV fusion inhibitors with pocket- or membrane-binding domain.Biochim Biophys Acta. 2012 Dec;1818(12):2950-7. doi: 10.1016/j.bbamem.2012.07.020. Epub 2012 Jul 31. Biochim Biophys Acta. 2012. PMID: 22867851
-
The Tryptophan-Rich Motif of HIV-1 gp41 Can Interact with the N-Terminal Deep Pocket Site: New Insights into the Structure and Function of gp41 and Its Inhibitors.J Virol. 2019 Dec 12;94(1):e01358-19. doi: 10.1128/JVI.01358-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31619552 Free PMC article.
-
HIV-1 gp41 fusion intermediate: a target for HIV therapeutics.J Formos Med Assoc. 2010 Feb;109(2):94-105. doi: 10.1016/S0929-6646(10)60029-0. J Formos Med Assoc. 2010. PMID: 20206833 Review.
-
Peptide and non-peptide HIV fusion inhibitors.Curr Pharm Des. 2002;8(8):563-80. doi: 10.2174/1381612024607180. Curr Pharm Des. 2002. PMID: 11945159 Review.
Cited by
-
Single-chain protein mimetics of the N-terminal heptad-repeat region of gp41 with potential as anti-HIV-1 drugs.Proc Natl Acad Sci U S A. 2014 Dec 23;111(51):18207-12. doi: 10.1073/pnas.1413592112. Epub 2014 Dec 8. Proc Natl Acad Sci U S A. 2014. PMID: 25489108 Free PMC article.
-
Peptide-Based Dual HIV and Coronavirus Entry Inhibitors.Adv Exp Med Biol. 2022;1366:87-100. doi: 10.1007/978-981-16-8702-0_6. Adv Exp Med Biol. 2022. PMID: 35412136 Review.
-
Trimeric heptad repeat synthetic peptides HR1 and HR2 efficiently inhibit HIV-1 entry.Biosci Rep. 2019 Sep 24;39(9):BSR20192196. doi: 10.1042/BSR20192196. Print 2019 Sep 30. Biosci Rep. 2019. PMID: 31477581 Free PMC article.
-
An effective strategy for recapitulating N-terminal heptad repeat trimers in enveloped virus surface glycoproteins for therapeutic applications.Chem Sci. 2016 Mar 1;7(3):2145-2150. doi: 10.1039/c5sc04046a. Epub 2015 Dec 3. Chem Sci. 2016. PMID: 29899942 Free PMC article.
-
A critical HA1 neutralizing domain of H5N1 influenza in an optimal conformation induces strong cross-protection.PLoS One. 2013;8(1):e53568. doi: 10.1371/journal.pone.0053568. Epub 2013 Jan 8. PLoS One. 2013. PMID: 23320093 Free PMC article.
References
-
- Eckert D. M., Kim P. S. (2001) Annu. Rev. Biochem. 70, 777–810 - PubMed
-
- Lu M., Blacklow S. C., Kim P. S. (1995) Nat. Struct. Biol. 2, 1075–1082 - PubMed
-
- Chan D. C., Fass D., Berger J. M., Kim P. S. (1997) Cell 89, 263–273 - PubMed
-
- Weissenhorn W., Dessen A., Harrison S. C., Skehel J. J., Wiley D. C. (1997) Nature 387, 426–430 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

