Transmembrane protein 147 (TMEM147) is a novel component of the Nicalin-NOMO protein complex
- PMID: 20538592
- PMCID: PMC2924024
- DOI: 10.1074/jbc.M110.132548
Transmembrane protein 147 (TMEM147) is a novel component of the Nicalin-NOMO protein complex
Abstract
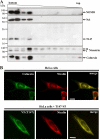
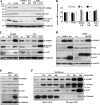
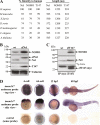
Nicastrin and its relative Nicalin (Nicastrin-like protein) are both members of larger protein complexes, namely gamma-secretase and the Nicalin-NOMO (Nodal modulator) complex. The gamma-secretase complex, which contains Presenilin, APH-1, and PEN-2 in addition to Nicastrin, catalyzes the proteolytic cleavage of the transmembrane domain of various proteins including the beta-amyloid precursor protein and Notch. Nicalin and its binding partner NOMO form a complex that was shown to modulate Nodal signaling in developing zebrafish embryos. Because its experimentally determined native size (200-220 kDa) could not be satisfyingly explained by the molecular masses of Nicalin (60 kDa) and NOMO (130 kDa), we searched in affinity-purified complex preparations for additional components in the low molecular mass range. A approximately 22-kDa protein was isolated and identified by mass spectrometry as transmembrane protein 147 (TMEM147), a novel, highly conserved membrane protein with a putative topology similar to APH-1. Like Nicalin and NOMO, it localizes to the endoplasmic reticulum and is expressed during early zebrafish development. Overexpression and knockdown experiments in cultured cells demonstrate a close relationship between the three proteins and suggest that they are components of the same complex. We present evidence that, similar to gamma-secretase, its assembly is hierarchical starting with the formation of a Nicalin-NOMO intermediate. Nicalin appears to represent the limiting factor regulating the assembly rate by stabilizing the other two components. We conclude that TMEM147 is a novel core component of the Nicalin-NOMO complex, further emphasizing its similarity with gamma-secretase.
Figures


References
-
- Selkoe D., Kopan R. (2003) Annu. Rev. Neurosci. 26, 565–597 - PubMed
-
- Haass C., Selkoe D. J. (2007) Nat. Rev. Mol. Cell Biol. 8, 101–112 - PubMed
-
- Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., Selkoe D. J. (1999) Nature 398, 513–517 - PubMed
-
- Yu G., Nishimura M., Arawaka S., Levitan D., Zhang L., Tandon A., Song Y. Q., Rogaeva E., Chen F., Kawarai T., Supala A., Levesque L., Yu H., Yang D. S., Holmes E., Milman P., Liang Y., Zhang D. M., Xu D. H., Sato C., Rogaev E., Smith M., Janus C., Zhang Y., Aebersold R., Farrer L. S., Sorbi S., Bruni A., Fraser P., St George-Hyslop P. (2000) Nature 407, 48–54 - PubMed
-
- Francis R., McGrath G., Zhang J., Ruddy D. A., Sym M., Apfeld J., Nicoll M., Maxwell M., Hai B., Ellis M. C., Parks A. L., Xu W., Li J., Gurney M., Myers R. L., Himes C. S., Hiebsch R., Ruble C., Nye J. S., Curtis D. (2002) Dev. Cell. 3, 85–97 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

