Dynamin-like MxA GTPase: structural insights into oligomerization and implications for antiviral activity
- PMID: 20538602
- PMCID: PMC2937866
- DOI: 10.1074/jbc.R110.145839
Dynamin-like MxA GTPase: structural insights into oligomerization and implications for antiviral activity
Abstract
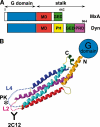
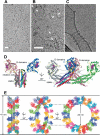
The interferon-inducible MxA GTPase is a key mediator of cell-autonomous innate immunity against a broad range of viruses such as influenza and bunyaviruses. MxA shares a similar domain structure with the dynamin superfamily of mechanochemical enzymes, including an N-terminal GTPase domain, a central middle domain, and a C-terminal GTPase effector domain. Recently, crystal structures of a GTPase domain dimer of dynamin 1 and of the oligomerized stalk of MxA (built by the middle and GTPase effector domains) were determined. These data provide exciting insights into the architecture and antiviral function of the MxA oligomer. Moreover, the structural knowledge paves the way for the development of novel antiviral drugs against influenza and other highly pathogenic viruses.
Figures
Similar articles
-
Structural basis of oligomerization in the stalk region of dynamin-like MxA.Nature. 2010 May 27;465(7297):502-6. doi: 10.1038/nature08972. Epub 2010 Apr 28. Nature. 2010. PMID: 20428112
-
Stalk domain of the dynamin-like MxA GTPase protein mediates membrane binding and liposome tubulation via the unstructured L4 loop.J Biol Chem. 2011 Oct 28;286(43):37858-65. doi: 10.1074/jbc.M111.249037. Epub 2011 Sep 7. J Biol Chem. 2011. PMID: 21900240 Free PMC article.
-
Human MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activity.J Interferon Cytokine Res. 2011 Jan;31(1):79-87. doi: 10.1089/jir.2010.0076. Epub 2010 Dec 19. J Interferon Cytokine Res. 2011. PMID: 21166595 Review.
-
Structure of myxovirus resistance protein a reveals intra- and intermolecular domain interactions required for the antiviral function.Immunity. 2011 Oct 28;35(4):514-25. doi: 10.1016/j.immuni.2011.07.012. Epub 2011 Sep 29. Immunity. 2011. PMID: 21962493
-
Interferon-induced mx proteins: dynamin-like GTPases with antiviral activity.Traffic. 2002 Oct;3(10):710-7. doi: 10.1034/j.1600-0854.2002.31003.x. Traffic. 2002. PMID: 12230469 Review.
Cited by
-
Molecular evolution and expression patterns of myxovirus resistance proteins in Lampetra japonica.Acta Biochim Biophys Sin (Shanghai). 2024 Mar 25;56(3):490-493. doi: 10.3724/abbs.2024019. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38400631 Free PMC article. No abstract available.
-
Characterization of the amino-terminal domain of Mx2/MxB-dependent interaction with the HIV-1 capsid.Protein Cell. 2014 Dec;5(12):954-7. doi: 10.1007/s13238-014-0113-5. Protein Cell. 2014. PMID: 25363729 Free PMC article. No abstract available.
-
GTPase activity of porcine Mx1 plays a dominant role in inhibiting the N-Nsp9 interaction and thus inhibiting PRRSV replication.J Virol. 2024 Apr 16;98(4):e0184423. doi: 10.1128/jvi.01844-23. Epub 2024 Mar 4. J Virol. 2024. PMID: 38436247 Free PMC article.
-
Autocrine activation of the IFN signaling pathway may promote immune escape in glioblastoma.Neuro Oncol. 2017 Oct 1;19(10):1338-1349. doi: 10.1093/neuonc/nox051. Neuro Oncol. 2017. PMID: 28475775 Free PMC article.
-
Two host microRNAs influence WSSV replication via STAT gene regulation.Sci Rep. 2016 Mar 31;6:23643. doi: 10.1038/srep23643. Sci Rep. 2016. PMID: 27029712 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

