Accumulation of isochorismate-derived 2,3-dihydroxybenzoic 3-O-beta-D-xyloside in arabidopsis resistance to pathogens and ageing of leaves
- PMID: 20538606
- PMCID: PMC2919129
- DOI: 10.1074/jbc.M109.092569
Accumulation of isochorismate-derived 2,3-dihydroxybenzoic 3-O-beta-D-xyloside in arabidopsis resistance to pathogens and ageing of leaves
Abstract
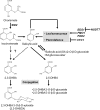
An intricate network of hormone signals regulates plant development and responses to biotic and abiotic stress. Salicylic acid (SA), derived from the shikimate/isochorismate pathway, is a key hormone in resistance to biotrophic pathogens. Several SA derivatives and associated modifying enzymes have been identified and implicated in the storage and channeling of benzoic acid intermediates or as bioactive molecules. However, the range and modes of action of SA-related metabolites remain elusive. In Arabidopsis, Enhanced Disease Susceptibility 1 (EDS1) promotes SA-dependent and SA-independent responses in resistance against pathogens. Here, we used metabolite profiling of Arabidopsis wild type and eds1 mutant leaf extracts to identify molecules, other than SA, whose accumulation requires EDS1 signaling. Nuclear magnetic resonance and mass spectrometry of isolated and purified compounds revealed 2,3-dihydroxybenzoic acid (2,3-DHBA) as an isochorismate-derived secondary metabolite whose accumulation depends on EDS1 in resistance responses and during ageing of plants. 2,3-DHBA exists predominantly as a xylose-conjugated form (2-hydroxy-3-beta-O-D-xylopyranosyloxy benzoic acid) that is structurally distinct from known SA-glucose conjugates. Analysis of DHBA accumulation profiles in various Arabidopsis mutants suggests an enzymatic route to 2,3-DHBA synthesis that is under the control of EDS1. We propose that components of the EDS1 pathway direct the generation or stabilization of 2,3-DHBA, which as a potentially bioactive molecule is sequestered as a xylose conjugate.
Figures






Similar articles
-
Salicylic acid antagonism of EDS1-driven cell death is important for immune and oxidative stress responses in Arabidopsis.Plant J. 2010 May 1;62(4):628-40. doi: 10.1111/j.1365-313X.2010.04178.x. Epub 2010 Feb 16. Plant J. 2010. PMID: 20163553
-
Salicylic acid 3-hydroxylase regulates Arabidopsis leaf longevity by mediating salicylic acid catabolism.Proc Natl Acad Sci U S A. 2013 Sep 3;110(36):14807-12. doi: 10.1073/pnas.1302702110. Epub 2013 Aug 19. Proc Natl Acad Sci U S A. 2013. PMID: 23959884 Free PMC article.
-
In-depth analysis of isochorismate synthase-derived metabolism in plant immunity: Identification of meta-substituted benzoates and salicyloyl-malate.J Biol Chem. 2024 Sep;300(9):107667. doi: 10.1016/j.jbc.2024.107667. Epub 2024 Aug 12. J Biol Chem. 2024. PMID: 39128721 Free PMC article.
-
Biosynthesis of salicylic acid in plants.Plant Signal Behav. 2009 Jun;4(6):493-6. doi: 10.4161/psb.4.6.8392. Epub 2009 Jun 12. Plant Signal Behav. 2009. PMID: 19816125 Free PMC article. Review.
-
Salicylic acid: an old hormone up to new tricks.Mol Plant Pathol. 2013 Aug;14(6):623-34. doi: 10.1111/mpp.12035. Epub 2013 Apr 28. Mol Plant Pathol. 2013. PMID: 23621321 Free PMC article. Review.
Cited by
-
Altering cold-regulated gene expression decouples the salicylic acid-growth trade-off in Arabidopsis.Plant Cell. 2024 Oct 3;36(10):4293-4308. doi: 10.1093/plcell/koae210. Plant Cell. 2024. PMID: 39056470 Free PMC article.
-
Long-term, sustained feeding by Asian citrus psyllid disrupts salicylic acid homeostasis in sweet orange.BMC Plant Biol. 2019 Nov 12;19(1):493. doi: 10.1186/s12870-019-2114-2. BMC Plant Biol. 2019. PMID: 31718546 Free PMC article.
-
The enterobactin biosynthetic intermediate 2,3-dihydroxybenzoic acid is a competitive inhibitor of the Escherichia coli isochorismatase EntB.Protein Sci. 2025 Jun;34(6):e70160. doi: 10.1002/pro.70160. Protein Sci. 2025. PMID: 40400396 Free PMC article.
-
Degradation of 2,3-dihydroxybenzoate by a novel meta-cleavage pathway.J Bacteriol. 2012 Aug;194(15):3851-60. doi: 10.1128/JB.00430-12. Epub 2012 May 18. J Bacteriol. 2012. PMID: 22609919 Free PMC article.
-
Muconic acid production from glucose using enterobactin precursors in Escherichia coli.J Ind Microbiol Biotechnol. 2015 May;42(5):701-9. doi: 10.1007/s10295-014-1581-6. Epub 2015 Feb 8. J Ind Microbiol Biotechnol. 2015. PMID: 25663483
References
-
- Pieterse C., Leon-Reyes A., Van der Ent S., Van Wees S. (2009) Nat. Chem. Biol. 5, 308–316 - PubMed
-
- Glazebrook J. (2005) Annu. Rev. Phytopathol. 43, 205–227 - PubMed
-
- Shah J. (2009) Curr. Opin. Plant Biol. 12, 459–464 - PubMed
-
- Bednarek P., Pislewska-Bednarek M., Svatos A., Schneider B., Doubsky J., Mansurova M., Humphry M., Consonni C., Panstruga R., Sanchez-Vallet A., Molina A., Schulze-Lefert P. (2009) Science 323, 101–106 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

